What Are The Signs And Symptoms Of Primary Hyperaldosteronism
Most people will not have disease-specific symptoms. The diagnosis is often made when people are incidentally found to have high blood pressure.
Patients with primary hyperaldosteronism are often diagnosed very late as the symptoms can be subtle. Most commonly, high blood pressure as a result of water and salt retention is found and a doctor will often suspect primary hyperaldosteronism if high blood pressure is not responding to multiple medications and/or if the patient is very young. About one-third of patients with primary hyperaldosteronism will also have a low blood level of potassium, which the kidney excretes in exchange for salt. Occasionally, this may cause symptoms such as cramps, weakness and excessive thirst.
Expression Of Key Molecules Of The Mineralocorticoid Response In Kidney Segments Involved In Acid
The MR is phosphorylated at various sites including at serine 483, a site that modifies binding of aldosterone and glucocorticoids to the receptor. Phosphorylation of S483 reduces affinity and activation of the receptor. In kidney in vivo, most MR is in the non-phosphorylated form except in intercalated cells that show a high degree of S483 phosphorylation . MR phosphorylation is decreased in states of elevated aldosterone such as in volume depletion . In contrast, in hyperkalemia, MR phosphorylation is increased and the receptor found mostly in the cytoplasm of intercalated cells whereas it is nuclear in principal cells. Angiotensin II may play a key role in regulation of the phosphorylation status of MR in intercalated cells and high angiotensin II reduces MR phosphorylation thereby providing a switch in the responsiveness of intercalated cells to aldosterone. The effects of angiotensin II may in part be mediated by the WNK4 kinase and the PP1 phosphatase .
The molecules mediating non-genomic rapid effects of aldosterone have not been identified to date. Possible candidates have been proposed and include GPR30 , a membrane-associated estrogen receptor. However, its role in physiological processes regulated by aldosterone in the kidney remained elusive .
How Does The Adrenal Hormone Aldosterone Regulate Kidney Function
This mineralocorticoid hormone produced by the zona glomerulosa plays a central role in regulating blood pressure and certain electrolytes . Aldosterone sends signals to the kidneys, resulting in the kidneys absorbing more sodium into the bloodstream and releasing potassium into the urine.
What is the function of cortisol and aldosterone?
Aldosterone helps control your blood pressure by managing the balance of potassium and sodium in your body. Cortisol works in conjunction with adrenaline and noradrenaline to help regulate your reaction to stress. Cortisol also helps regulate your metabolism, sugar levels, and blood pressure.
You May Like: What Tea Is Good For Kidney Function
Pkd3 Regulation Of Enac And Renal Na+ Transport
To date, the role of PKD3 in the kidney remains unknown. PKD3 is unlike the other two isoforms whereby it is present in the nucleus as well as the cytoplasm in unstimulated cells. Currently, investigations of PKD3-dependent signaling pathways in the kidney are lacking, while studies in other tissues and cell types have been reliant on the use of non-specific pharmacological inhibitors or the use of small interfering RNA . We have used both siRNA and CRISPR/Cas knockdown of PKD3 in M1-CCD cells to obtain some insights into a potential role for PKD3 in the renal transport of sodium. Preliminary data show that PKD3 is primarily localized in the cytoplasm and perinuclear region and translocates to the nucleus under aldosterone treatment or low Na+ diet .
PKD3 knockdown resulted in reduced genomic expression of the ENaC subunit and SGK. Long-term treatment with aldosterone produced a reduced sodium transport rate in PKD3 suppressed cells compared to wild-type CCD. Knockdown of PKD3 did not interfere with PKD1 nor PKD 2 non-genomic signaling in response to aldosterone nor did it significantly affect basal Na+ transport rates. It thus appears that PKD3 is a genomic signal pathway for aldosterone regulation of ENaC and SGK and may synergize and reinforce with the non-genomic PKD1 and PKD2 modulation of ENaC trafficking and membrane stabilization .
Figure 9.
Are There Any Side
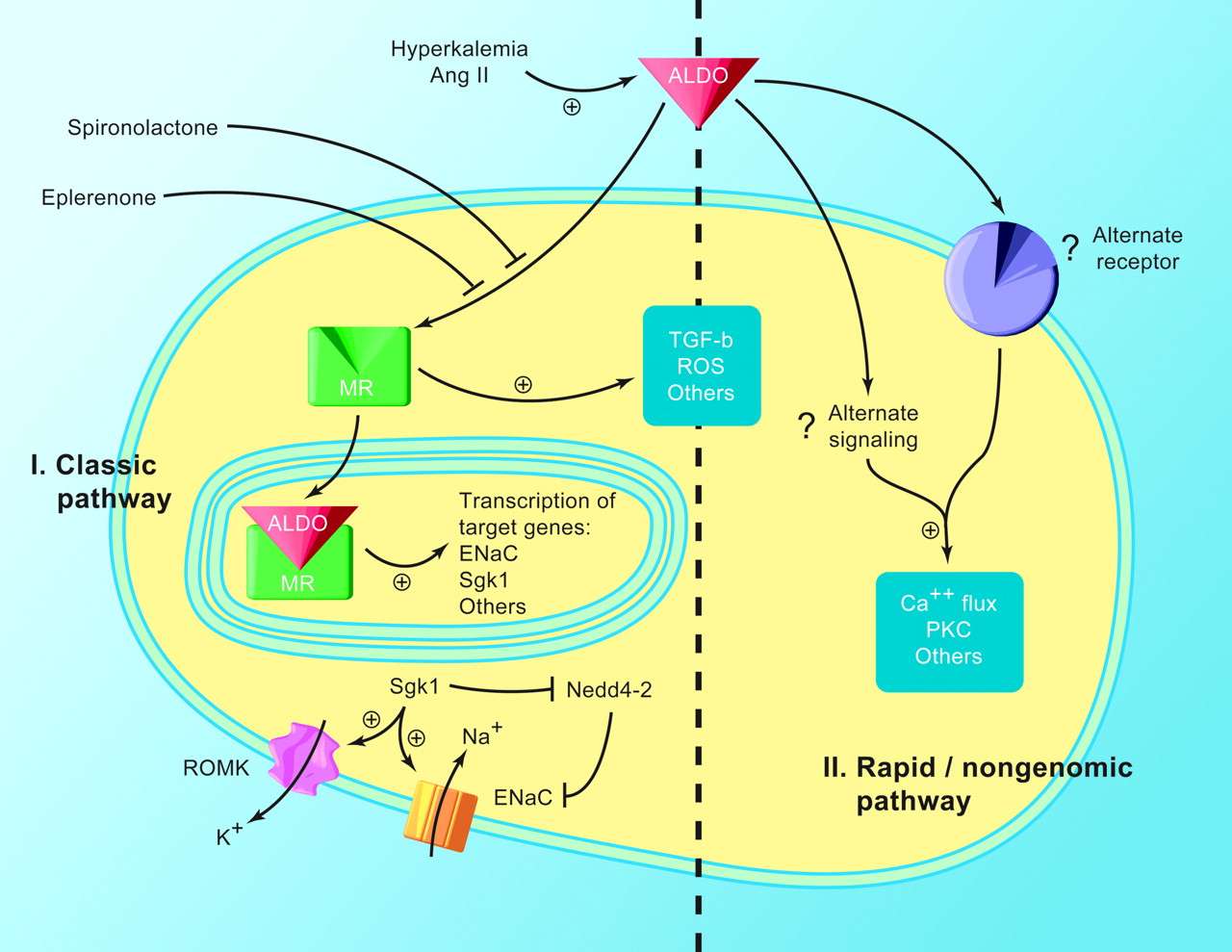
The medication most commonly used to treat primary hyperaldosteronism can cause high blood potassium and low blood salt as it blocks the action of aldosterone. It can affect periods in women and in men it can also cause impotence and enlargement of breast tissue, as it blocks the action of testosterone. Eplerenone is an alternative drug that works through the same mechanism as spironolactone but without the sex hormone side-effects however eplerenone is much more expensive.
Pregnant women or those of child bearing age would usually not be offered spironolactone as it can have serious effects on the developing baby.
Don’t Miss: Does Diet Soda Cause Kidney Stones
Testing For Aldosterone Hypersecretion
Hypersecretion of aldosterone by adrenal masses is extremely rare, with only approximately 1% of adrenal adenomas responsible for Conn syndrome . Nevertheless, data demonstrate that nearly 5% of newly hypertensive patients may harbor an aldosterone-secreting adenoma . Indeed, testing of hypertensive patients with adrenal lesions for hyperaldosteronemia is clinically recommended. Testing of nonhypertensive patients, however, currently is not recommended. The section on primary aldosteronism describes the physiologic rationale for each test. In this section, we review the practical implications of evaluating adrenal incidentalomas for excess aldosterone secretion.
In the past, low serum potassium level has been used as a screening tool to assess for the presence of aldosterone hypersecretion. Despite this prior teaching, contemporary series reveal that less than 40% of patients with hyperaldosteronism exhibit hypokalemia .Today the screening test of choice for Conn syndrome is the ratio of morning plasma aldosterone to renin . An ARR of 20 along with a concomitant aldosterone concentration above 15ng/mL is indicative of Conn syndrome . The concurrent elevated aldosterone level appears important for cases in which the ARR is elevated simply because of a low renin level. The test’s characteristics have not been fully defined however, sensitivities and specificities in the 90th percentile have been reported .
Aldosterone And Thyroid Health
I have written numerous articles and blog posts on adrenal health in the past, and usually I focus on the adrenal hormones cortisol and DHEA. But rarely do I talk about aldosterone, and so I figured Id dedicate a post to this important hormone. Aldosterone is a mineralcorticoid, and like cortisol and DHEA it is also secreted by the adrenal glands. As a result, if someone has adrenal insufficiency then this not only can lead to a decrease in cortisol levels, but it can also lead to a decrease in aldosterone as well. When someone has problems with blood pressure and/or water retention the hormone aldosterone is usually involved, as Ill discuss in this article.
In order to have a better understanding of the importance of aldosterone, Id like to briefly talk about the renin-angiotensin-aldosterone system . This system helps to regulate blood pressure and fluid imbalance. As a result, if someone has high or low blood pressure, or problems with water retention, then this system will be involved.
Aldosterone, Blood Pressure Regulation, and Water Retention
Primary vs. Secondary Hyperaldosteronism
Can Taking Too Much Licorice Root Affect Aldosterone Levels?
What Can Cause Low Aldosterone Levels?
How Does Aldosterone Relate To Thyroid Health?
Are There Natural Solutions For Aldosterone Imbalances?
Also Check: Grapes Kidney Stones
Molecular Mechanisms Of Enac Trafficking Regulated By Pkd1
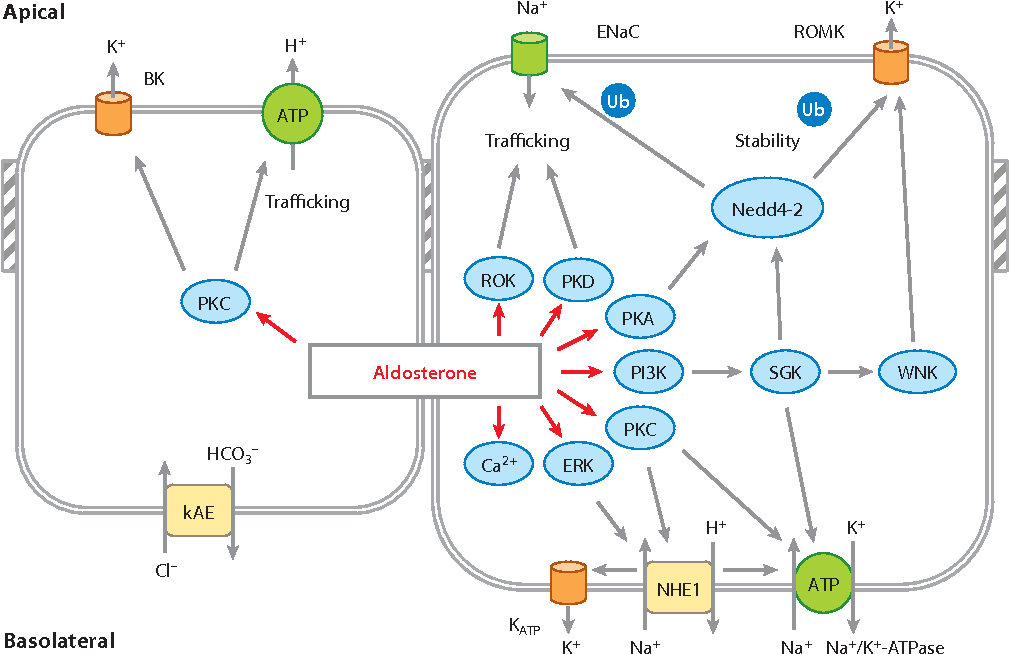
Membrane-localized ENaC is subject to constant recycling. The inclusion of ENaC into the apical membrane is a prerequisite for its ubiquitination and retrieval into the subapical pool or its degradation by the proteasome. Nedd4-2 interacts with ENaC through a C-terminal PY internalization motif to facilitate ENaC ubiquitination. The surface expression of ENaC may be equally regulated by deubiquitination by DUBs and ubiquitination by Nedd4-2. However, only PKD1 and PKD2 isoforms provide the composite signaling pathway for the basal control and acute stimulatory effect of aldosterone that influences cellular trafficking dynamics controlling ENaC and Na/K pump membrane targeting, insertion, stabilization, and retrieval .
Figure 6.
ENaC and Na/K pump membrane targeting under the control of PKD1. PKD1 regulates the correct targeting of ENaC and Na+/K+-ATPase to the apical and basolateral membranes, respectively. Knocking down PKD1 expression in CCD cells results in the defective membrane sorting of ENaC and Na/K pump such that their membrane expression polarity is inverted.
What Causes Primary Hyperaldosteronism
Hyperaldosteronism may be due to either diffuse swelling/overgrowth affecting the adrenal gland, in one or both glands or, a small tumour within the gland . In both cases, there is excessive secretion of aldosterone. These tumours are invariably small, benign, and do not spread or invade other areas in the way malignant tumours can.
Rarely, the condition is hereditary so called glucocorticoid or dexamethasone-suppressible hyperaldosteronism. Rarer still, large adrenal carcinomas may secrete aldosterone. In all conditions, the feedback system fails and aldosterone secretion continues despite a low blood renin level.
You May Like: What Does Flomax Do For Kidney Stones
Cellular Mechanism Of The Classical Model
The classical actions of aldosterone are mediated by intracellular receptors that translocate to the nucleus upon ligand binding. The activated steroid receptor modulates gene expression by functioning as a transcription factor. Two distinct molecular mechanisms, depicted in , define the actions of nuclear receptors on gene expression. The classical mechanisms involve activation and repression via direct interaction with DNA binding sites . More recent studies of glucocorticoid:receptor function have established a novel complementary mechanism by which steroids affect transcription . In this process, transcription interference or synergy are mediated by protein-protein interactions between activated steroid receptors and other factors. In this second mechanism, the steroid receptor does not physically bind DNA, even though the mechanism does impinge upon gene expression.
Classical Model Of Aldosterone Action
shows an idealized cell model of the classical aldosterone-sensitive epithelial cell, the principal cell of the renal collecting duct. Monolayers of these cells serve two primary and related functions: acting as barriers separating the internal and external environments and allowing absorption of Na+ followed by water. The barrier function is primarily dependent on the lipid composition of the apical membrane and the formation of high-resistance, electrically tight monolayers, an emergent property of the apical plasma membrane and the junctional complexes coupling these cells. The transport function is regulated by aldosterone.
FIG. 1Model of the genomic actions of aldosterone in epithelial cells. Asterisks denote final effectors of aldosterone involved in Na+ and K+ reabsorption and secretion, respectively. The aldosterone-induced proteins serum and glucocorticoid-inducible kinase , corticosteroid hormone-induced factor , and Kirsten Ras increase the activity and/or no. of these transport proteins during the early phase of action. During the late phase of aldosterone action, expression levels of transport proteins themselves increase. ENaC, epithelial Na+ channel Hsp, heat shock protein MR, mineralocorticoid receptor GR, glucocorticoid receptor SRE, steroid response element 11-HSD2, 11–hydroxysteroid dehydrogenase type 2.
Recommended Reading: Is Apple Cider Vinegar Bad For Kidney Disease
What Is Primary Hyperaldosteronism
Aldosterone is a hormone produced by the adrenal glands located just above each kidney. Aldosterone helps to control the amount of fluid in the body by affecting how much salt and water the kidney retains or excretes. Aldosterone production from the adrenal gland is regulated by another hormone called renin. Renin is produced by specialised cells in the kidney that detect when the body lacks salt. The kidney secretes renin which stimulates the adrenal glands to release aldosterone. The kidney detects an increase in aldosterone in the bloodstream and responds by retaining extra salt rather than excreting it in the urine. As the body regains the salt it needs, the level of renin in the bloodstream drops and therefore the amount of aldosterone in the blood also falls, meaning more water is excreted in the urine. This is an example of a feedback system.
Hyperaldosteronism refers to any state where there is excessive or inappropriate levels of aldosterone in the bloodstream. In primary hyperaldosteronism, aldosterone secretion is inappropriately high for the level of body salt and blood volume regardless of the renin level in the blood . Secondary hyperaldosteronism occurs when the kidney produces too much renin. This is often seen in patients with chronic low blood volume such as in cardiac, liver or renal disease the kidney mistakes the low blood supply for dehydration and produces excess renin.
Does Renin Increase Blood Pressure
Not exactly. On its own, renin doesnt affect your blood pressure. Instead, it works together with angiotensin and aldosterone to accomplish this. Angiotensin narrows your blood vessels and aldosterone causes your kidneys to retain water and salt. This increases the amount of fluid in your body and raises your blood pressure.
You May Like: Is Watermelon Bad For Your Kidneys
Pkd1 Pkd2 Sgk1 And The Regulation Of Enac Membrane Trafficking And Stability
The upregulation of SGK1 is the earliest transcriptional and translational response that is elicited by aldosterone, whereas PKD1 and PKD2 are the earliest activated kinases in the non-genomic response to aldosterone in the regulation of ENaC trafficking and stabilization in the apical membrane stabilization. The interactions between aldosterone-stimulated PKD1, PKD2, and SGK have not been investigated however, certain predictions to guide future research can be proposed and tested . Given that PKD1, PKD2, and SGK would be expected to enhance ENaC trafficking, insertion, and stability and to reduce ENaC retrieval and degradation following aldosterone treatment, it would be expected that some cross talk and coordination would exist between these kinases in their kinetics of activation, subcellular localization, and cooperativity. However, from previous work it appears that SGK does not play an essential role in basal or Na+ deprivation-induced renal Na+ transport or ENaC activity, whereas PKD1 and PKD2 expression and activation are absolutely critical for the maintenance of basal and aldosterone-stimulated ENaC function and transepithelial sodium transport.
Figure 8.
Control Of Aldosterone Release From The Adrenal Cortex
The role of the reninangiotensin system
Angiotensin is involved in regulating aldosterone and is the core regulation. Angiotensin II acts synergistically with potassium, and the potassium feedback is virtually inoperative when no angiotensin II is present. A small portion of the regulation resulting from angiotensin II must take place indirectly from decreased blood flow through the liver due to constriction of capillaries. When the blood flow decreases so does the destruction of aldosterone by liver enzymes.
Although sustained production of aldosterone requires persistent calcium entry through low-voltage-activated Ca2+ channels, isolated zona glomerulosa cells are considered nonexcitable, with recorded membrane voltages that are too hyperpolarized to permit Ca2+ channels entry. However, mouse zona glomerulosa cells within adrenal slices spontaneously generate membrane potential oscillations of low periodicity this innate electrical excitability of zona glomerulosa cells provides a platform for the production of a recurrent Ca2+ channels signal that can be controlled by angiotensin II and extracellular potassium, the 2 major regulators of aldosterone production.Voltage-gated Ca2+ channels have been detected in the zona glomerulosa of the human adrenal, which suggests that Ca2+ channel blockers may directly influence the adrenocortical biosynthesis of aldosterone in vivo.
The plasma concentration of potassium
Adrenocorticotropic hormone
The role of sympathetic nerves
Recommended Reading: Soda Causes Kidney Stones
Role Of Aldosterone In Acid
In animal models and patients with reduced kidney function, renal acidosis develops and may contribute to the further decline in renal function . In an earlier stage of reduced nephron numbers, acid-base balance is maintained and may depend on elevated angiotensin II levels . In later stages of CKD enhanced aldosterone secretion may be promoted by stimulated endothelin production due to accumulation of acid in kidney and other tissues. Treatment of acidosis with alkali substitution reduces aldosterone in these patients . Blockade of MR appears to slow down the rate of loss of GFR in a rat model of CKD . How aldosterone alone or in conjunction with acidosis and other factors contributes to decline of renal function is an open question.
Aldosterone Is A Mineralocorticoid That Increases Na+ Retention And K+ Excretion
Aldosterone is a steroid hormone produced in the outermost layer of the adrenal gland, the zona glomerulosa. Its secretion is controlled predominantly by angiotensin II and plasma . Increasing plasma increases aldosterone secretion. Other stimuli include decreased plasma and adrenocorticotrophic hormone . Aldosterones chemical structure is shown in Figure 7.6.9.
Figure 7.6.9. Chemical structure of aldosterone.
Aldosterone acts according to the classical steroid hormone model in which the hormone binds to cytosolic receptors and is transferred to the nucleus where it binds to nuclear receptors that stimulate the transcription of specific genes. Aldosterone increases Na+ reabsorption and increases K+ secretion in cells in the cortical collecting duct. Evidence suggests that aldosterone increases the activity of the basolateral Na+K+-ATPase, ENaC, and the apical K+ channel, ROMK .
Lorenza González-Mariscal, … Miguel Quirós, in, 2010
Don’t Miss: Aleve Effect On Kidneys
Experimental Murine Ckd Model
The experimental mouse study was approved by the Animal Welfare Committee of the Austrian Federal Ministry of Science and Research and was undertaken in strict accordance with the guidelines for animal care . All experiments were performed in 1416-weeks old male C57Bl/6 mice. Mice were kept at 24°C with a 12/12-h light/dark cycle and had ad libitum access to food and water. CKD was surgically induced via 5/6-Nx under isoflurane anesthesia as previously described . Buprenorphine and metamizole were applied as analgesic drugs before surgery. Postsurgical pain management involved application of metamizole for 3 days, as well as buprenorphine for at least 1 day. In the first surgery, 2/3 of the left kidney were removed. Seven days later, mice underwent total nephrectomy for their right kidney. Because it is well known that C57Bl/6 mice are relatively resistant to the development of CKD after 5/6-Nx, mice were fed a diet containing 2.0% calcium, 1.25% phosphorus, and 20% lactose , which accelerates renal injury due to the high phosphate content . 5/6-Nx C57Bl/6 mice on this phosphate-enriched diet show an about 5060% reduction in glomerular filtration rate as assessed by creatinine clearance 8 weeks postsurgery . In Sham mice left and right kidneys were exposed and repositioned before closing the flank incision. Mice were exsanguinated from the abdominal Vena cava under general anaesthesia for serum collection 4, 8, or 12 weeks postsurgery. Samples were stored at 80°C.