Renal Control Of Plasma Hco3
The kidneys have the predominant role of regulating the systemic HCO3 concentration and hence, the metabolic component of acid-base balance. This function of the kidneys has two components: reabsorption of virtually all of the filtered HCO3 and production of new HCO3 to replace that consumed by normal or pathologic acids. This production or generation of new HCO3 is done by net acid excretion. In other words, the kidneys make new HCO3 by excreting acid.
Because HCO3 is freely filtered at the glomerulus, approximately 4.5 mol HCO3 is normally filtered per day . Virtually all of this filtered HCO3 is reabsorbed, with the urine normally essentially free of HCO3. Seventy to eighty percent of this filtered HCO3 is reabsorbed in the proximal tubule the rest is reabsorbed along more distal segments of the nephron .
Relative HCO3transport along the nephron. Most of the filtered HCO3 is reabsorbed in the proximal tubule. Virtually no HCO3 remains in the final urine. CCD, cortical collecting duct DT, distal convoluted tubule IMCD, inner medullary collecting duct TAL, thick ascending limb.
Loss of alkali in the urine in the form of HCO3 decreases the amount of net acid excretion or new HCO3 generation. Loss of organic anions, such as citrate, in the urine represents the loss of potential alkali or HCO3. However, in humans, the loss of these organic anions is not usually quantitatively significant in wholebody acid-base balance .
What Ph Should Urine Be To Prevent Kidney Stones
Can alkaline water give you kidney stones?
I dont think anyone should be drinking alkaline water without speaking with their doctor first, says Dr. Timothy Hlavinka. In rare cases, weve seen alkaline water consumption coincide with the development of serious kidney stones. Hlavinka recommends plain water be consumed instead.
What Causes Metabolic Acidosis
Healthy kidneys have many jobs. One of these jobs is to keep the right balance of acids in the body. The kidneys do this by removing acid from the body through urine. Metabolic acidosis is caused by a build-up of too many acids in the blood. This happens when your kidneys are unable to remove enough acid from your blood.
Read Also: Can My Kidneys Hurt From Drinking
Metabolism Of Dietary Weak Acids
Fruits and vegetables, particularly citrus fruit, contain many weak acids and the salts of those acids . We all know that citrus juice is acidic, with some fruit juices having a pH below 4.0. Interestingly, metabolism of these acidic substances alkalinizes the blood, sometimes called the fruit juice paradox. The complete oxidation of the protonated form of an organic acid to CO2 and water is acid-base neutral, no different in principle than the oxidation of glucose. However, the complete oxidation of the base form adds bicarbonate to the body, that is, organic anions are precursors of bicarbonate. One can think of metabolizing an organic base anion as taking a hydrogen ion from the body fluids to protonate the anion, thus converting it to a neutral acid, and then oxidizing the acid. The loss of the hydrogen ion, as emphasized above, adds bicarbonate. This loss of hydrogen ions greatly exceeds the number of free hydrogen ions present in the original fruit juice. Although its pH is low, there is far more base than free hydrogen ions. Before oxidation, the mixture is acidic, but on complete oxidation to CO2 and water, the result is addition of base.
Other Factors Which Affect Renal Acid
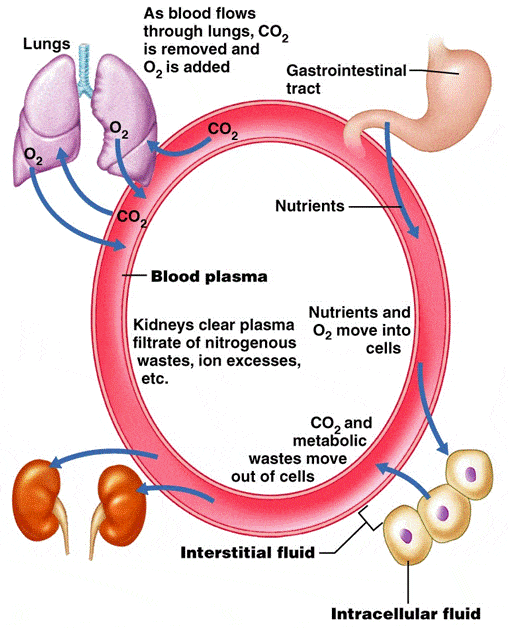
There a few other peripheral matters to attend do, with regards to renal acid-base handling. As all must surely have noticed by now, the movements of sodium and potassium are key to renal acid-base balance management. And as sodium handling and potassium handling are tightly regulated by various neurohormonal actors, it stands to reason that these actors will have some indirect effect on acid-base handling as well.
Read Also: How Fast Can Kidney Stones Form
What Happens If The Ph In Your Pool Is Too High
One of the most frequent problems is when a pools pH gets too high. The pH is a measure of the waters balance between acidity and alkalinity. If the pH isnt properly balanced, problems can occur. Water with a pH thats too high also can cause skin rashes, cloudy water and scaling on pool equipment.
Renal Response To Acid
When systemic acid-base disorders occur, the kidneys respond by appropriately altering RNAE. Thus, with acidosis RNAE excretion increases, whereas RNAE decreases with alkalosis. Most of our understanding of the adaptive response of the kidneys to acid-base disorders has come from using models of metabolic acidosis. The following section describes our current understanding of how RNAE increases in this setting.
With acidosis RNAE increases. This response includes a reduction, if not elimination, of all HCO3 from the urine and increases in TA and NH4+ excretion. The key elements of this response include the stimulation of H+ and HCO3 transport along the nephron, increased ammoniagenesis, and increased availability of urinary buffers .
Acidosis can directly reduce the intracellular pH of renal tubular cells. This cellular acidosis has been shown to stimulate NHE3 activity via allosteric mechanisms, change transporter kinetics due to altered H+ gradients across membranes, and cause exocytotic insertion of transporters into the plasma membrane from intracellular stores . It is uncertain as whether these effects of intracellular acidosis are mediated by pH alone or secondary to the activation of intracellular regulatory pathways or mechanisms. However, it is now clear that other factors also mediate the renal response to acidosis. For example, endothelin-1 and glucocorticoids clearly play a role in the stimulation of renal H+ and HCO3 transport during acidosis.
| Proximal tubule |
Also Check: Is An 8mm Kidney Stone Large
What Are The Symptoms Of Being Too Acidic
When your body fluids contain too much acid, its known as acidosis. Acidosis occurs when your kidneys and lungs cant keep your bodys pH in balance. Many of the bodys processes produce acid.Respiratory acidosis fatigue or drowsiness. becoming tired easily. confusion. shortness of breath. sleepiness. headache.
How Does The Respiratory System Reduce Acidosis
The lungs and the kidneys are the major organs that help regulate your bloods pH. The lungs remove acid by exhaling CO2, and the kidneys excrete acids through the urine. The kidneys also regulate your bloods concentration of bicarbonate .
What are the main ways the kidneys maintain the acid-base balance of the blood quizlet?
The kidneys help maintain the acidbase balance by excreting hydrogen ions into the urine and reabsorbing bicarbonate from the urine.
Recommended Reading: How Often Is Kidney Disease Misdiagnosed
Expanded Conceptual Framework Integrating Interactions Between Ph Homeostasis And Progression Of Chronic Kidney Disease
Most probably, certain effects of pH on kidney function act independently of each other. However, independent effectors may synergize into further kidney injury, and some others may actually be dependent on each other. Here, we propose a conceptual framework with multiple known factors involved in acidbase-dependent progression of chronic kidney disease and how they would evolve from early to late stages of CKD . In this figure, we also list key open questions related to multiple steps of this framework.
Fig. 3
Conceptual framework how chronic kidney disease, inflammation, and deranged metabolism form a vicious cycle involving metabolic acidosis as an engine. Nephron loss and impaired renal function reduce kidney capacity of eliminating acids and generating new bicarbonate which leads to accumulation of acids in the organism. Renal responses to acidosis exacerbate inflammation and deranged metabolism that ultimately reduce kidney function and kidney capacity of keeping pH homeostasis. Steps of this network are shown in continuous black boxes, and open questions related to each of these steps are shown next to it in dashed black boxes. Inflammation and metabolism domains are artificially delimited in different colors as some of these steps may belong to both domains
Role Of Angiotensin Ii
The hormone that has the greatest stimulatory effect on JHCO3 is angiotensin II , applied at a low dosage to either the luminal or basolateral surface . When applied at a high dosage to either surface, however, AngII actually reduces JHCO3 .
We examined the effect low-dosage and of high-dosage AngII on the response of JHCO3 to alterations in B . We found that low-dosage AngII, added to either the lumen or the bath, tends to shift the JHCO3-versus-B relationship to the left, so that lower levels of B tend to stimulate HCO3 reabsorption. Conversely, high-dosage AngII, added to either the lumen or the bath, tends to blunt the JHCO3-versus-B relationship.
Endogenous luminal AngII plays at least a critical permissive role in the tubules response to alterations in B. It would be interesting to know whether basolateral CO2 somehow accentuates the endogenous AngII system, perhaps by increasing the secretion of AngII, increasing the density or the sensitivity of apical AT1 receptors, or enhancing downstream signaling from the AT1 receptor to the acid-base transporters.
Also Check: Will Donating A Kidney Shorten My Life
The Kidneys Help Maintain Acid
Learning Objective
-
Describe the role of the kidney in acid-base balance
Key Points
- The kidneys maintain homeostasis through excretion of waste products.
- Acidosis causes more bicarbonate to be reabsorbed from tubular fluid while collecting ducts secrete more hydrogen to generate more bicarbonate and more NH3 buffer is formed.
- Alkalosis causes the kidney to excrete more bicarbonate as there is reduced secretion of hydrogen ions and more ammonium is excreted.
Terms
-
Pertaining to the kidneys.
Example
Full Text
Within the human body, fluids such as blood must be maintained within the narrow range of 7.35 to 7.45, making it slightly alkaline. Outside the range, pH becomes incompatible with life proteins are denatured and digested, enzymes lose their ability to function, and the body is unable to sustain itself.
To maintain this narrow range of pH the body has a powerful buffering system. Acidââ¬âbase imbalances that overcome this system can be compensated in the short term by changing the rate of ventilation.
The Role Of The Kidney In Acid
The kidneys have two main ways to maintain acid-base balance – their cells reabsorb bicarbonate HCO3 from the urine back to the blood and they secrete hydrogen H+ ions into the urine.
Our kidneys filter blood continuously by distributing the blood that comes into the kidney to millions of tiny functional units called .
Each is made up of the glomerulus, or a tiny clump of , where blood filtration begins.
When blood passes through a glomerulus, about one-fifth of the plasma leaves the glomerular capillaries and goes into the renal tubule.
Reabsorption of the good stuff—water and electrolytes—and leaving behind the bad stuff—waste products and acid— is the job of the the .
The renal tubule is a structure with several segments: the , the U- shaped with a thin descending and a thick ascending limb, and the , which winds and twists back up again, before emptying into the collecting duct, which collects the final urine.
Each of these tubules is lined by brush border cells which have two surfaces.
One is the apical surface that faces the tubular lumen and is lined with microvilli, which are tiny little projections that increase the cells surface area to help with solute reabsorption.
The other is the basolateral surface, which faces the peritubular capillaries, which run alongside the .
So with , as the filtrate leaves the glomerulus, it first goes through the .
You May Like: How Long Is Kidney Stone Surgery
Good Ph Balance = Good Health
Generally, health is maintained when the body’s fluids have their normal pH measurement. Different fluids have different measurements. For instance the blood and tissue fluid normally have a measurement of 7.35 to 7.45, while urine usually has a measurement of 5 to 6. Once they stay within these ranges, a state of health usually exists. When they move out of these ranges, however, sickness or even death could result.
Maintaining or restoring a balanced pH is not possible when the body is loaded with acid toxins. Acid imbalances compromise the efficient functioning of all cells and organs, leading to premature deterioration and degeneration of health.
NOTE: All degenerative diseases and disease-causing organisms only develop in an acidic environment.
Nutritional chemistry reveals that raw fruits and vegetables provide the best source of nutrients that helps to maintain the constant pH balance of the body’s biological systems. When heat is applied to these foods, through cooking, or preservatives and refined sugars are added to them, most of their nutritional properties are destroyed and they become more acidic. When we consume these foods in their acidic state, we contribute to acid imbalances within the body’s biochemical systems. This places greater burden on our buffer systems to regulate these imbalances that can lead to their malfunctioning, over time.
What Are The Signs Of Acidosis Or Alkalosis
Signs and Symptoms Acute metabolic acidosis may also cause an increased rate and depth of breathing, confusion, and headaches, and it can lead to seizures, coma, and in some cases death. Symptoms of alkalosis are often due to associated potassium loss and may include irritability, weakness, and muscle cramping.
Also Check: Can Calcium Cause Kidney Stones
Metabolic Acidosis In Kidney Transplant Recipients
Interestingly, metabolic acidosis occurs in kidney transplant recipients at higher eGFR levels when compared to patients with CKD . This finding suggests that there may be transplant-specific mechanisms involved, and it is further supported by the fact that metabolic acidosis in KTRs typically presents with the features of renal tubular acidosis , such as normal anion gap metabolic acidosis, compared to high anion gap acidosis in patients with CKD . Among the transplant-specific features, calcineurin inhibitors may be of great importance. Data from animal and human studies demonstrated that both cyclosporine and tacrolimus may affect tubular function including recent findings about the role of pendrin in the pathogenesis of distal RTA . In addition, other elements, such as immunological factor associated with allograft rejection, donor-associated factors , and dietary factors , may also contribute to the development of metabolic acidosis in KTRs. In a recent study, we investigated the impact of metabolic acidosis and its therapy on molecular changes in renal biopsies of KTRs via RNA sequencing and immunofluorescence . Our data demonstrated that metabolic acidosis in kidney transplant recipients is associated with changes in the renal transcriptome and protein expression of genes mostly involved in acidbase transport and cell energy metabolism . These changes were partly reconstituted by alkali therapy .
The Effects Of Acid Imbalance
The normal pH balance of blood plasma is approximately 7.4. If it falls below 7.35, a state of acidosis exists and if it rises above 7.45, a state of alkalosis exists. A person will only survive for a few hours if their pH balance is below 7.0 or above 7.7. It is quickly fatal if it falls below 6.8 or above 8.0. In a state of acidosis, the central nervous system becomes depressed and this causes symptoms such as confusion, disorientation, and coma. In a state of alkalosis, the skeletal muscles are over stimulated causing muscle spasms, convulsions, or respiratory paralysis.
Acid-base imbalances fall into two categories – respiratory and metabolic. Respiratory acidosis occurs when there is an accumulation of carbon dioxide, which lowers the Potential Hydrogen. On the other hand, Respiratory alkalosis results from hyperventilation, in which carbon dioxide is eliminated faster than it is produced.
Metabolic acidosis can result from increased production of organic acids, such as lactic acid in anaerobic fermentation and ketone bodies in alcoholism and diabetes mellitus. It can also result from chronic diarrhea or overuse of laxatives, or from ingestion of acidic drugs such as aspirin. Metabolic alkalosis is not as common as metabolic acidosis. It can result from overuse of bicarbonates such as antacids or intravenous bicarbonate solutions. It can also result from the loss of stomach acids due to chronic vomiting.
Also Check: Does Ascorbic Acid Cause Kidney Stones
Role Of The Proximal Tubule In Acid
Schematic illustration of acid-base handling in the renal proximal tubules. H+ ions secreted by the proximal tubules via Na+/H+ exchanger 3 combine with filtered bicarbonate to form H2CO3. Through the action of carbonic anhydrase IV and II, the filtered bicarbonate gets reabsorbed back into the blood via electrogenic sodium bicarbonate cotransporter 1 on the basolateral side. Upon stimulation, several proteins such as calcium-sensing receptor , proline-rich tyrosine kinase 2 , and G protein-coupled receptor family C group 5 member C can influence NHE3 activity, thereby modulating acid-base homeostasis. TWIK-related acid-sensitive K+ channel 2 is essential for maintaining basolateral membrane potential, and its absence affects bicarbonate reabsorption in the proximal tubules.
The Ph Balance Of The Body Is Maintained By The Kidneys
The term pH balance refers to a measurement of the degree to which a substance is acidic or alkaline. In chemistry, pH is the acronym for “Potential Hydrogen” and it is the measurement of acidity.
An acid is any chemical that releases hydrogen ions in solution. The Potential Hydrogen scale was invented in 1909 to measure the acidity of beer. The scale extends from 0.0 to 14.
A solution with a measurement of 7 is neutral solutions measuring below 7 are acidic and solutions measuring above 7 are alkaline . To put this scale into perspective, it is important to note that a change of one whole number represents a 10-fold change in hydrogen ion concentration. For example, a solution with a measurement of 4 is 10 times as acidic as one with a measurement of 5 and 100 times as acidic as one with a measurement of 6.
Slight disturbances of the acid-balance of the body’s fluids can seriously disrupt biological functions. Maintaining the pH balance of the body’s fluids is one of the key functions of the kidneys. Blood, for example, normally has a measurement ranging from 7.35 to 7.45. Deviations from this range can cause fainting, paralysis, or even death.
You May Like: How Can A Person Survive With One Kidney