Specific Drugs And Therapeutic Uses
Aliskiren is a renin inhibitor that was approved for the treatment of hypertension by the U.S. FDA in 2007. Aliskiren is an orally active nonpeptide drug with a half-life of about 24 hours, and is dosed once per day. Because of its relatively long half-life, it takes about 2 weeks of dosing to achieve a near maximal antihypertensive effect. It is metabolized by the liver and excreted by the kidneys. Normal therapeutic concentrations of aliskiren reduce plasma renin activity by 50-80%. It is effective as monotherapy. When used in conjunction with thiazide diuretics or ARBs, the antihypertensive effects are additive.
The Kidneys Are Stimulated To Produce Renin True Or False
True
Explanation:
Kidneys are defined as a pair of bean-shaped organ present in the abdominal cavity of mammals, reptiles, and birds responsible for excretion of urine.
Renin is a peptide hormone which is secreted by the kidneys from granular cells that are present in the juxtaglomerular apparatus. The secretion of renin stimulation is based on decrease in sodium chloride in the juxtaglomerular apparatus by the macula densa, fall in arterial blood pressure in the arterial vessels by baroreceptors and sympathetic nervous system activity is through beta 1 adrenergic receptors.
Hence, the given statement is true.
C) by a decrease in the blood pressure
Read Also: Is Cranberry Juice Good For The Liver
Effects Of Dopamine On Renin Release
The effects of dopamine on renin release are variable, depending on the species and the experimental model. Intrarenal infusion of dopamine in the conscious dog causes a dose-dependent increase in renin release, which is attenuated by the DA2 receptor antagonist sulpiride. Similarly, in both the isolated perfused rat kidney and dispersed rat renal cortical cell preparations, dopamine stimulates renin release via the activation of DA2 receptors. In humans, the intravenous infusion of dopamine has been shown either to have no effect on plasma renin activity or to increase PRA. In contrast, oral administration of the DA2 receptor agonist bromocriptine, in humans, causes a decrease in PRA. It has been hypothesized that the stimulatory effects of dopamine on renin release may relate to a direct postsynaptic effect on the renal juxtaglomerular cells and that the inhibitory actions of dopamine on renin release may be presynaptic. Further studies are essential to clarify the actions of dopamine on renin release, especially in humans.
Victoria F. Norwood, … R. Ariel Gomez, in, 2004
You May Like: What Test Is Used To Detect Kidney Stones
Renin In The Juxtaglomerular Cell
Despite the importance of JG cells in regulating blood pressure and electrolyte homeostasis, characterization of their molecular and cellular properties are incomplete. Based on the presence of myofilaments in their cytoplasm, it was postulated that JG cells derive from smooth muscle lineages in the kidney . However, later studies suggested that JG cells originate from metanephric mesenchyme, migrating and incorporating into the developing afferent arterioles of the embryonic kidney, but only later acquiring smooth muscle markers .
JG cells are mainly defined by their unique localization in the JG region of the renal afferent arteriole and their ability to synthesize and secrete renin . They have a large nucleus, hypertrophic rough endoplasmic reticulum and distinct Golgi apparatus. JG cells carry two types of secretory granules: large electron-dense, mature granules containing active renin, Ang peptides, and cathepsins, along with smaller, electron-lucent proto-granules containing active renin and pro-renin . JG cells have a distinct transcriptional signature compared to other renal cell types, consisting of arterial, interstitial cell, and pericyte markers, as well genes associated with endocrine and contractile functions .
Major Renal Effects Of The Raas
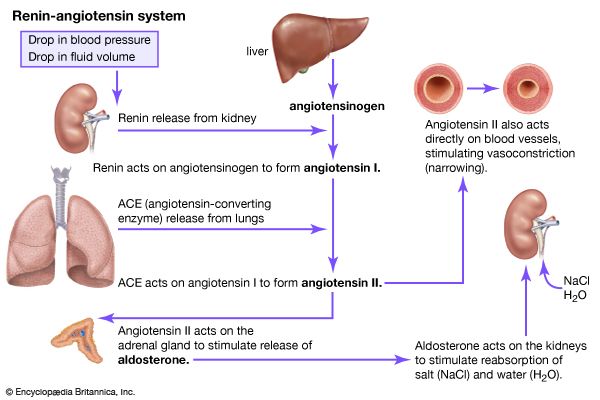
Angiotensinogen is synthesized by hepatocytes and released into the plasma . Renin a protease produced by the myoepithelioid cells of the afferent arteriole of the renal glomerulus cleaves the angiotensinogen to ang-1. Renin is rapidly secreted in response to a decrease in blood pressure as detected by baroreceptors, a decrease in the sodium concentration delivered to the distal tubules, and activation of the sympathetic nervous system . The conversion enzyme is a carboxy-peptidase that cleaves plasma ang-1 to ang-2. Ang-2 stimulates the AT-1 and AT-2 receptors .
Fig. 1.
Schematic representation of the role of the RAAS modulation in kidney disease. TGF-, transforming growth factor-beta.
The AT-1 receptors are expressed over the entire length of the nephron but predominantly on the proximal circumvoluted tubules. Ang-2 essentially exerts a vasoconstrictive action on the efferent arteriole of the glomerular capillaries, increasing the glomerular capillary hydraulic pressure. Ang-2 is the key mediator in the renal blood flow and glomerular filtration rate autoregulation. In the event of a decrease in systemic arterial pressure, renal blood flow and glomerular filtration are aimed to be maintained by the vasodilation of the afferent arteriole and vasoconstriction of the efferent arteriole through ang-2 production .
Recommended Reading: Can Blood Thinners Cause Kidney Problems
Sympathetic Activity In Patients With Crf
Clinical studies demonstrating increased concentrations of plasma catecholamines and enhanced sensitivity to norepinephrine were the first indicators of sympathetic hyperactivity in patients with CRF., These early observations were substantiated by a pronounced hypotensive effect in response to adrenergic inhibition with clonidine or debrisoquine. In 1992, Converse et al. first reported that muscle sympathetic nerve activity, as assessed by clinical microneurography, is increased in patients who have ESRD and undergo hemodialysis. Most interesting, bilaterally nephrectomized patients had a sympathetic drive comparable to control subjects without renal failure and also had lower BP. This was the first clinical finding pointing to a role for the diseased kidneys in sympathetic activation. Initially, uremia-related toxins were suggested to be responsible for afferent renal sympathetic nerve traffic however, increased sympathetic nerve activity is already evident in compensated CRF, and correction of uremia by renal transplantation does not result in normalization of sympathetic nerve activity. Furthermore, sympathetic activation is present in models of acute renal damage and renal ischemia, in the absence of uremia, and increased noradrenaline secretion rates are found in patients with nephrotic syndrome and in individuals with hypertension and autosomal dominant polycystic kidney disease despite normal renal function.
The Raa System And Covid
The Coronavirus disease 2019 , or SARS-CoV-2, led to a global outbreak that affected nearly 200 million people worldwide as of July 2021. The disease is associated with severe complications in people who have pre-existing cardiovascular diseases, such as hypertension and diabetes.
The renin-angiotensin system plays an important role in the COVID-19 infectious disease process.
The SARS-CoV-2 uses angiotensin-converting enzyme 2 as a “receptor” and cellular entry point to infect a wide range of cells in the body. More specifically, ACE 2, which is embedded in the surfaces of cells, is recognized by spike proteins on the COVID-19 virus. This recognition leads to a lock-and-key relationship that opens the door for the virus to enter.
Dr_Microbe / Getty Images
Don’t Miss: Can Kidneys Heal On Their Own
Local Factors Influencing Renin Secretion
Several neurotransmitters and neuropeptides, such as norepinephrine, dopamine,, calcitonin gene-related peptide, vasoactive intestinal peptide and pituitary adenylate cyclase-activating peptide have been found to stimulate renin secretion by stimulating the cAMP pathway, whereas neuropeptide Y inhibits renin secretion by inhibiting cAMP formation. Among these factors the physiological relevance is best characterized for norepinephrine which binds to 1-receptors on renin-secreting cells. Norepinephrine-induced renin secretion occurs already at low sympathetic renal nerve activity and thus temporally precedes renal vascoconstriction which occurs at higher sympathetic output to the kidneys.
The tubular macula densa cells and the preglomerular endothelial cells produce the same autacoids, namely nitric oxide and prostaglandins, albeit by different mechanisms. Nitric oxide is generated by nitric oxide synthases NOS-1 and NOS-3 in macula densa and in endothelial cells, respectively. Prostaglandins are generated by cyclooxygenases COX-2 and COX-1 in macula densa and in endothelial cells, respectively . Both nitric oxide and prostaglandins such as PGI2 and PGE2 act stimulatory on renin secretion in an additive fashion. Prostaglandins stimulate cAMP formation, whereas nitric oxide attenuates cAMP degradation via inhibition of cAMP-phosphodiesterases.
How Is Angiotensin Controlled
An increase in renin production occurs if there is a decrease in sodium levels and a decrease in blood pressure, which is sensed by the kidneys. In addition, low blood pressure can stimulate the sympathetic nervous system to increase renin production, which results in increased conversion of angiotensinogen to angiotensin I, and so the cycle continues.
The reninangiotensin system is also activated by other hormones, including oestrogen rel=nofollow> oestrogen and thyroid hormones. On the other hand, natriuretic peptides can impede the reninangiotensin system in order to increase sodium loss in the urine.
Don’t Miss: Is Kidney Stones A Disease
Nitric Oxide And The Cyclic Gmp Pathway In Regulation Of Renin
Increases in intracellular levels of cGMP in JG cells can be triggered by various stimuli such as nitric oxide and atrial natriuretic peptide . Depending on the circumstances, they can either inhibit or stimulate renin release . NO stimulation in vivo consistently results in increased renin release , and the endothelial form of nitric oxide synthase is required for renin cell recruitment . It has been suggested that NO stimulates renin release through the formation of cGMP, which can inhibit PDEs, thereby attenuating cAMP hydrolysis . In parallel, cGMP can potentially suppress renin secretion through activation of cGMP regulated protein kinase type II and inhibition of renin by cGMP agonists is lost in mice with targeted disruption of cGKII . Levels of cGKII are subject to regulation in the JG cell , but the impact of this regulation on control of renin release has not been established.
Physiological Control Of Renin Secretion In Vivo
The before mentioned findings on the mechanisms controlling renin secretion were mostly obtained in isolated preparations or in laboratory animals under specific conditions that do not provide direct information about the relative contribution of the different pathways to the integrative control of renin secretion in vivo.
The secretion of renin in the normal healthy organism is in the low range, meaning that there is no major regulatory range left to further suppress renin synthesis and renin secretion beyond the normal situation. Conversely, renin synthesis and renin secretion are strongly stimulated if blood pressure, salt homeostasis and/or glomerular filtration are threatened, supporting the notion of an emergency function of the RAAS.
Recommended Reading: Does A Kidney Stone Hurt All The Time
Effects Of Ang Ii On Potassium Secretion
Increases in plasma potassium concentration directly increase the release of aldosterone from the adrenal cortex, independent of the actions of ANG II. Aldosterone increases potassium secretion in the distal segments of the nephron. Aldosterone stimulates potassium secretion by increasing the following:
Sodium-potassium ATPase protein and activity at the basolateral membrane of distal nephron segments, which increases the electrochemical driving force for potassium entry into the cell and increases the secretion of potassium across the apical membrane.
Activity of enzymes of the tricarboxylic acid cycle.
Production of ATP to be used as an energy source for the sodium-potassium ATPase pump.
The expression and activation of epithelial sodium channels, which enhances sodium entry into the cell and increases lumen negativity, which then increases the secretion of potassium.
Permeability of the apical membrane to potassium by increasing the number of potassium channels and enhancing the activation of these potassium channels.
Therefore, increasing apical secretion of potassium increases potassium excretion by the kidneys.
Dont Miss: Can A Kidney Infection Cause Diarrhea
Addressing Underlying Health Conditions
If you have low renin levels, your doctor will recommend treatment and monitoring based on the underlying cause.
For example, the following conditions must be resolved in order to normalize renin levels:
- High aldosterone read more about aldosterone.
- Cushings Syndrome
This list is not exhaustive.
Once the underlying cause of low levels is under control, you may talk to your healthcare provider about lifestyle changes and complementary approaches that may help.
Below are some approaches to bring up with your doctor. These are typically recommended to people with low renin and high blood pressure.
Don’t Miss: Is Apple Cider Vinegar Good For Kidney Infection
Processing And Secretion Of Renin In Jg Cells
Renin is initially synthesized as a pre-pro-renin protein. After cleavage of the pre-fragment, pro-renin is transferred to the Golgi. From there, pro-renin can be immediately secreted by the constitutive pathway or sorted to the dense-core secretory granules for regulated exocytosis . It appears that release of pro-renin through the constitutive pathway depends directly on the levels of renin synthesis per se, including levels of transcription per cell and the total number of renin-producing cells . Acute stimulation of renin release involves exocytosis of mature renin secretory granules that contain active renin only whereas chronic stimulation results in release of both pro-renin and renin into the circulation .
Studies in renin knockout mice suggest that glycosylation is crucial for pro-renin sorting into the dense core secretory granules. Ren1 and Ren2 proteins differ in their glycosylation patterns and studies using knock out mice for Ren1 or Ren2 gene show differences in their renin processing. Indeed, deletion of the Ren2 gene alone did not cause an apparent phenotype . In contrast, the kidneys in Ren1 deficient mice showed a very low number or complete absence of dense-core renin vesicles . It has been postulated that glycosylation differences may be responsible for these phenotypic differences .
What Hormones Do The Kidneys Produce
The kidneys make two main hormones, vitamin D and erythropoietin.
Vitamin D is essential for a number of different functions in the body. Most of the vitamin D that is in the blood is inactive and it is modified by the kidney and other tissues to activate it. Active vitamin D stimulates the uptake of calcium from food, is important for the maintenance of healthy bones and also helps to regulate the response of the immune system to infection.
Erythropoietin is produced when oxygen levels in the blood are low. It acts in bone marrow to stimulate the production of mature red blood cells, to maintain healthy oxygen levels in our tissues.
The kidneys also produce prostaglandins, hormone-like substances, made from lipid . The substances are one way in which the production of renin is stimulated. Renin is an enzyme, also produced by the kidneys, that plays an important role in the reninangiotensinaldosterone hormonal system, which helps to control blood pressure. In addition to making hormones, the kidneys also respond to a number of hormones including vitamin D, aldosterone, prostaglandins, cortisol, parathyroid hormone and calcitonin.
Don’t Miss: How To Pass Kidney Stones Fast
Nacl Reabsorption At The Macula Densa
The second major pathway for physiological regulation of renin is the macula densa mechanism whereby cells at the macula densa sense a reduction in chloride ions in the filtrate of the distal tubule, triggering renin release . In this circumstance, release of renin and the consequent generation of Ang II are believed to serve as a mechanism for enhancing renal sodium reabsorption in states of fluid volume depletion. The anatomical association of the macula densa with the JG apparatus stimulated the first speculation by Goormaghtigh of its physiological function . The macula densa is made up of specialized epithelial cells at the terminal portion of the thick ascending limb. Their basolateral membrane is in contact with glomerular mesangial cells, which, in turn, are contiguous with granular cells in the JG apparatus . The role of the macula densa in renin regulation was initially hypothesized by Vander in 1967 and there is now general consensus that this mechanism provides a control of renin secretion that is directly determined by NaCl delivery to the distal nephron . Moreover, several studies indicate that chloride flux through the NKCC2 regulates the signaling pathways linked to renin secretion . Increased chloride delivery to the macula densa inhibits, whereas reduced chloride delivery stimulates renin release .
Prostanoids And Nitric Oxide
A possible mediator function of prostaglandins is also conceivable for the stimulation of renin secretion by renal artery stenosis. It has been found that the vascular expression of COX-2 and the production of prostaglandin E2 are increased in stenotic kidneys in parallel with increased renin secretion. Inhibition of prostaglandin formation in those patients attenuates renin secretion from the stenotic kidneys.
Read Also: Is Ibuprofen Bad For Liver Or Kidneys
Elevated Ace In Hemodialysis Patients
In 1979, Patel et al. performed one of the first and best known evaluations of the levels of ACE in HD patients . They studied 19 patients with CKD on long-term HD and 19 controls, and found that ACE activity was increased in 58% of the patients . They proposed that these increased serum ACE levels might be secondary to increased pulmonary vascular area in patients with renal failure, because ACE was thought to be produced only in the lungs. In 1982, Muira et al. confirmed these results but proposed a different mechanism. They noted that the ACE activity of patients with CKD on regular HD was higher than that of age-matched controls , and they also found higher ACE levels in patients on HD for longer than 3 years and in patients with diabetic nephropathy. It is important to note that elevated ACE also occurs in DM . Muira et al. found no relationships of enzyme activity with age and sex . Based on studies reporting accelerated atherosclerosis in patients on maintenance HD, they speculated that advanced endothelial damage could lead to elevated serum ACE in HD patients, because vascular endothelial cells of the lungs and kidneys produce most of the ACE.
Localization And Ultrastructure Of Renin
Because of their localization and cuboid-like appearance, these cells are commonly termed juxtaglomerular epithelioid cells. The cuboid form results from huge intracellular vesicles , which are dependent on the production of glycosylated prorenin. Although transcription factors and micro-RNAs relevant for the specific phenotype of these cells have been suggested, the exact nature and the origin of these cells is still unknown. Circumstantial evidence suggests that the renin-producing cells might differentiate from pericytes which are probably also precursors of preglomerular vascular cells and glomerular mesangial cells. In the normal adult kidney renin-producing cells appear just at the junction between these two cell types, suggesting that these cells remain in an intermediate differentiation state between vascular smooth muscle cells on the one side and mesangial cells on the other. Compatible with this idea is the capability of preglomerular vascular smooth muscle cells and extraglomerular mesangial cells to reversibly switch on and off the renin gene expression even in the adult kidney, that leads to an increase or decrease in the number of renin-producing cells .
Don’t Miss: What’s The Signs Of A Kidney Infection