Hypoxia In Aki And Ckd
Kidney tissue hypoxia has been detected in multiple forms of AKI, including postoperative AKI, sepsis, and drug-induced nephropathy . Experimental models of sepsis demonstrated a significant decrease in the proportion of peritubular capillaries that showed normal blood flow . The mechanism of this abnormal microcirculation is incompletely understood, but it has been proposed that increased production of inducible nitric oxide synthase and cytokine-induced endothelial damage may have played a role . In addition, unbalanced oxygen supply and demand also contributes to the development of hypoxia in AKI. For example, administration of radiocontrast agents increased oxygen consumption for tubular transport, and inhibition of transport activity with furosemide reversed contrast agentinduced medullary hypoxia . It must be noted, however, that solely improving medullary oxygenation by furosemide does not necessarily translate into better kidney outcomes .
Isolation Of Inner Medullary Collecting Duct Cells
To test whether cells in the inner medulla activate HIF under physiological hypoxia, inner medullary collecting duct cells were isolated from HRE-Luctransgenic rats and exposed to 2% O2, as previously described . In brief, renal medulla were excised, cut into small pieces, and digested with 0.2% collagenase and 0.2% hyaluronidase . After the enzymatic digestion, cells were isolated by three short low-speed centrifugations and were seeded on six-well culture plates precoated with gelatin. With this method, more than 90% of obtained cells were collecting duct cells, as confirmed by positive immunostaining for aquaporin-2 . Then, cells were cultured with Dulbecco’s modified Eagle medium containing 5% fetal calf serum at 37°C under a humidified atmosphere of 5%CO2/95% air, grown to confluence, and exposed to hypoxia for 6 hours. Assuming the physiological medullary partial pressure of oxygen to be approximately 15 mmHg , we subjected IMCD cells to 2% oxygen . The HIF activity was estimated by measuring the amount of the transgene mRNA by real-time PCR, as described above. For practical reasons, only rats in the YO group were used in this study.
The Effects Of Hif Activation In Ckd
HIF accumulation has been observed in multiple forms of CKD , and there is much debate about its pathophysiological role. Although HIF is generally considered to promote cell survival under hypoxic conditions, some studies suggest that long-term HIF activation may bring about its harmful effects, such as fibrogenesis and inflammation. For example, HIF was demonstrated to induce profibrotic factors , and stabilization of HIF in kidney proximal tubules, by genetic deletion of Vhl, promoted tubulointerstitial fibrosis in a subtotal nephrectomy model . An opposing view is that HIF activation in CKD is in fact insufficient to achieve optimal cytoprotection. This notion is supported by studies that demonstrated the effects of indoxyl sulfate, a representative uremic toxin, on HIF activity. Administration of indoxyl sulfate suppressed nuclear accumulation of HIF-2 and subsequent production of EPO . Indoxyl sulfate also inhibited HIF-1 activity by inducing the expression of transcriptional repressors of HIF-1 . Additionally, oxidative stress and the diabetic milieu impair HIF functions . These findings led to the idea that therapeutic strategy to activate HIF may facilitate adaptive response to hypoxia and prevent CKD progression.
Preclinical studies to investigate the effects of HIF-related gene manipulation on the progression of various models of CKD
You May Like: What Type Of Epithelial Tissue Lines Kidney Tubules
Quantification Of The Transgene And Hif
Messenger RNA expression of the transgene , HIF- isoforms , and several of known HIF-regulated genes was quantified by real-time polymerase chain reaction . RNA was isolated from the renal cortex by using ISOGEN and 1 g of template was reverse-transcribed . One-twentieth of the complementary DNA product was used as a template for subsequent quantification. Using iQ SYBR Green Supermix , PCRs were performed on an iCycler under the following conditions: initial denaturation of the template at 94°C for 15 minutes, 40 cycles of amplification at 94°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. Data collection and analysis were performed using iCycler iQ Optical System software , and the amount of mRNA of interest was corrected for that of -actin. The sets of primers used are as follows: Luciferase: forward : 5-CGTTTCCAAAAAGGGGTTGC-3, reverse : 5-GAAGGACTCTGGCACAAAATCG-3 HIF-1: fw: 5-GTTTACTAAAGGACAAGTCACC-3, rv: 5-TTCTGTTTGTTGAAGGGAG-3 HIF-2: fw: 5-GTCACCAGAACTTGTGC-3, rv: 5-CAAAGATGCTGTTCATGG-3 EPO: fw: 5-TACGTAGCCTCACTTCACTGCTT-3, rv: 5-GCAGAAAGTATCCGCTGTGAGTGTTC-3 VEGF : fw: 5-TTACTGCTGTACCTCCAC -3, rv: 5-ACAGGACGGCTTGAAGATA-3 GLUT1: fw: 5-CAGTTCGGCTATAACACCGGTGTC-3, rv: 5-ATAGCGGTGGTTCCATGTTT-3 heme oxygenase-1 : fw: 5-TCTATCGTGCTCGCATGAAC-3, rv: 5-CAGCTCCTCAAACAGCTCAA-3 and -actin: fw: 5-CTTTCTACAATGAGCTGCGTG-3, rv: 5-TCATGAGGTAGTCTGTCAGG-3 .
Therapeutic Opportunities Beyond Renal Anemia
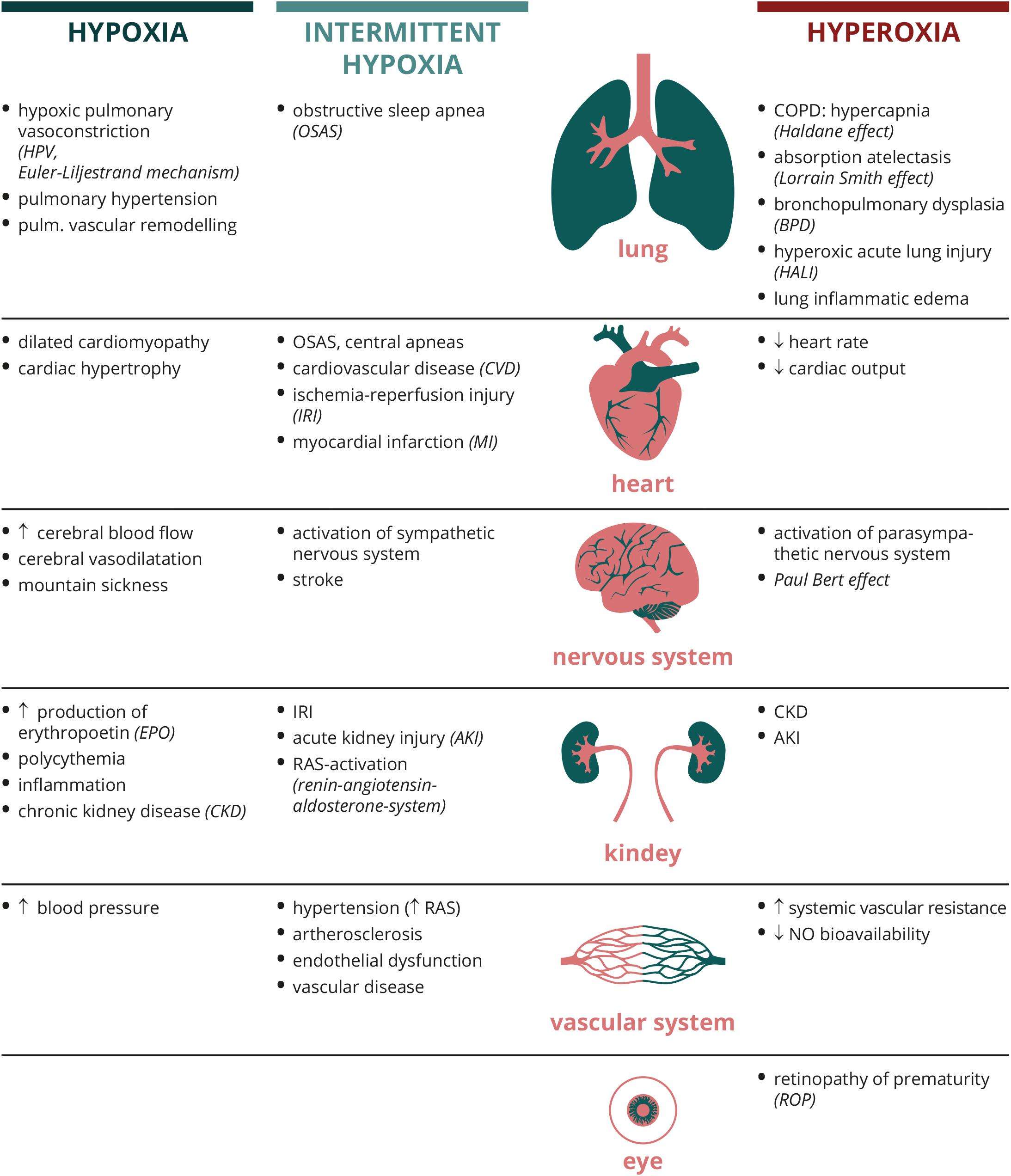
The effects of hypoxia and oxygen-dependent signaling on the kidney are broad. Although acute changes in renal pO2 are frequently encountered in hospitalized patients and often result in AKI, not infrequently requiring renal replacement therapy, the cellular and molecular consequences of chronic or intermittent hypoxia on the kidney are less obvious. Some insights into the effects of subacute or chronic hypoxic signaling on healthy and diseased kidneys can be gained from studies of humans living at high altitude and from patients with renal tumors caused by mutations in the O2 sensing machinery, such as patients with mutations in the von Hippel-Lindau tumor suppressor that result in constitutive HIF activation.
In the acute setting, HIF has been shown to mediate the effects of ischemic preconditioning, and pharmacologic HIF activation protects from ischemia-reperfusion injury in animal models of ARF. While the use of pharmacologic HIF activation in the prevention of acute renal injury is supported by preclinical studies, its role in CKD is debated animal models of progressive kidney injury support both renoprotective and injury-promoting roles. Recent data using the remnant kidney model furthermore indicate that renoprotection is dependent on the timing of pharmacologic HIF activation.
You May Like: How Often Is Kidney Disease Misdiagnosed
Respiratory Effects Of Hypoxaemia
Effect on respiratory drive: As the relationship of arterial oxygen and alveolar ventilation is detailed elsewhere, this chapter will not digress extensively on the ventilatory response to hypoxemia, only to say that:
- Sensors: peripheral chemoreceptors at the carotid glomus and aortic arch
- Afferents: vagus nerve and glossopharyngeal nerve
- Controller: central medullary and pontine respiratory control centres
- Efferents: phrenic nerve and spinal innervation of respiratory skeletal muscles
- Effectors: diaphragm, intercostal muscles, scalenes and abdominal muscles
Effect on gas exchange: There are two mechanisms by which hypoxia affects gas exchange. One is a brutally stupid effect related to the difference in partial pressures between the capillary blood and the alveolar content. Low partial pressure of oxygen in the alveolus causes a decrease in the diffusion rate of oxygen purely because the concentration gradient between the alveolus and the capillary is smaller. Consider a preposterous thought experiment where the venous PO2 is 40 mmHg, and the alveolar PO2 is also suddenly made 40 mmHg. Clearly, no diffusion of any sort is going to take place.
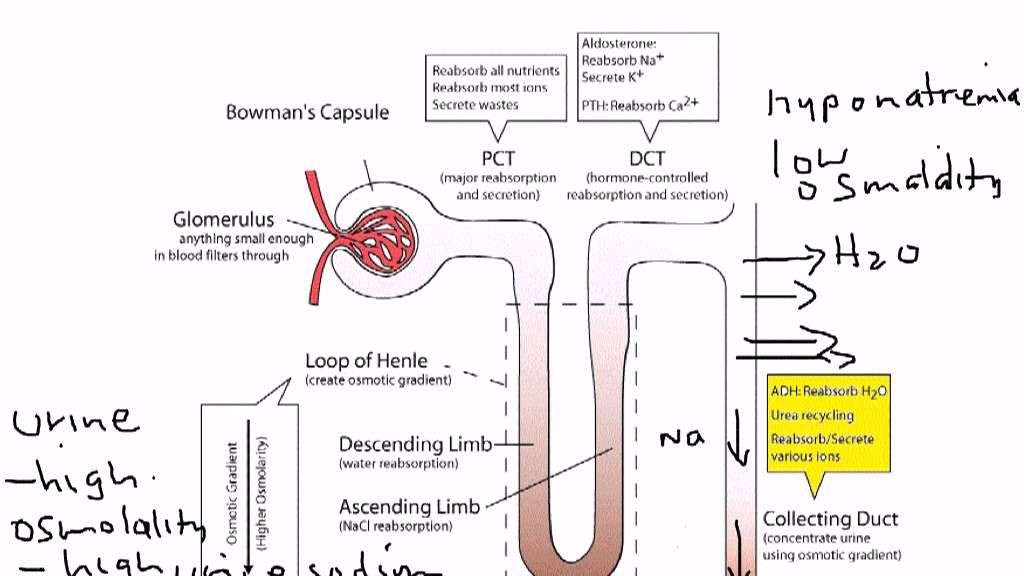
Susceptibility Of The Kidney To Hypoxia
In 1960, Aukland and Krog reported heterogeneous oxygen tension within the kidney of healthy dogs, which was much lower in the medullary region compared with the cortex. The subsequent studies also reported that oxygen tension in the medulla was 1020 mm Hg, whereas that in the cortex was 3060 mm Hg . However, it was later recognized that the tissue oxygenation was affected by general anesthesia, which reduces renal blood flow . The medullary oxygen tension of healthy, nonanesthetized sheep was reported to be 3040 mm Hg, which was similar to that in the cortex . It should be noted, however, that the basal tissue perfusion in the medulla was demonstrated to be significantly less, and the decrease in perfusion and oxygenation was greater in the medullary region during partial renal artery occlusion . This finding suggests that its inadequate ability to maintain oxygen homeostasis renders the renal medulla particularly susceptible to hypoxia under pathological conditions. In addition, studies using a hypoxic marker, pimonidazole, demonstrated positive staining in the medulla and corticomedullary regions in kidneys of healthy rats . These observations led to the concept that the renal medulla is relatively hypoxic, even if there is no apparent decrease in tissue oxygen tension.
Read Also: Is Clonidine Safe For Kidneys
Hif Stabilization As A Treatment Of Ckd
Given that renal hypoxia is a final common pathway in CKD progression and that HIF activation in CKD appears to be suboptimal, HIF stabilization presents a reasonable target for CKD. This concept has been supported by numerous studies using several animal models of CKD . Recently, Palm and colleagues demonstrated that the administration of cobalt ameliorated kidney damage in streptozotocin-induced diabetic rats, which was accompanied by increased oxygen tension in the kidney. However, these studies have several limitations. Polycythemia, which is frequently observed in studies with chronic HIF stabilization, may have confounding effects on the kidney. Moreover, cobalt, which has been used to stabilize HIF in numerous studies, has systemic effects, such as body weight loss, with < 30 % overlap of gene expression changes by treatment with cobalt and hypoxia . Therefore, the usefulness of HIF stabilization in CKD should be investigated in animal and clinical studies by using specific PHD inhibitors, which are being developed as therapeutics against renal anemia.
Maladaptation To Hypoxia During Ckd Progression
As discussed above, advanced renal hypoxia is observed in animal and human CKD. Despite several controversies, HIF accumulation has been shown to occur at certain stages during CKD, which is expected to protect against hypoxia . Nevertheless, in many CKD patients, kidney hypoxia does not improve and is rather aggravated, and renal function shows a sustained decline, resulting in ESKD. To date, several possible mechanisms have been proposed, which are discussed below .
Fig. 2
Maladaptation to hypoxia during CKD progression. HIF accumulation occurring at certain stages during CKD is expected to protect the kidney against hypoxia . Nevertheless, in many CKD patients, kidney hypoxia does not improve, resulting in ESKD via several mechanisms . Further details are explained in the text. HIF hypoxia-inducible factor, DM diabetes mellitus, VEGF vascular endothelial growth factor, EPC endothelial progenitor cell, UCP2 uncoupling protein 2
Read Also: Which System Do Kidneys Belong To
Hif And Profibrotic Gene Expression
Hypoxia induces collagen I, decreases matrix-metallopeptidase 2, and increases TIMP-1 in renal epithelial cells . Hypoxia can also act synergistically with TGF-1 in the regulation of certain hypoxia-responsive genes such as VEGF , endoglin , and EPO . Synergistic effects between hypoxia and TGF-1 have furthermore been demonstrated with regard to the production of collagens . These observations and the finding that several genes which play critical roles in renal fibrogenesis are direct HIF-1 targets suggest that increased HIF activity is likely to play an important role in the pathogenesis of tubulointerstitial fibrosis through direct transcriptional regulation of specific profibrotic genes and/or through enhancement of TGF-1 signaling. Synergistic interaction between SMAD3, a downstream effector of TGF-1, and HIF-1 has been suggested by Sanchez-Elsner et al. as a possible mechanism in the transcriptional regulation of VEGF. Although a direct role for HIF has yet to be demonstrated, hypoxia also increases SMAD3 mRNA levels and promotes the thrombospondin-dependent release of latent TGF-, thus activating TGF- signaling . Whereas the concept of direct regulation of profibrotic genes by HIF-1 is straightforward and consistent with the canonical hypoxia response, the interplay of HIF-1 and TGF- signaling appears to be complex and is more difficult to understand.
Molecular O2 Sensors In The Kidney: More Than Just Hif
Key components of cellular O2 sensing are Fe and 2-oxoglutarate dependent oxygenases . These enzymes belong to a larger family of proteins in humans, there are > 60 members that couple the oxidative decarboxylation of 2OG to various chemical processes, which include collagen synthesis and fatty acid metabolism. In mammals, these reactions appear to be limited to hydroxylation and demethylation initiated by hydroxylation and produce succinate and CO2 . The 2OG oxygenases control hypoxic signaling by catalyzing the hydroxylation of specific proline residues within the oxygen-dependent degradation domain of HIF- under normoxia. HIFs are pleiotropic oxygen-sensitive, heterodimeric transcription factors that have key roles in the cellular adaptation to hypoxia, and regulate a multitude of biologic processes, which include erythropoiesis and iron metabolism, anaerobic glucose metabolism, angiogenesis, growth, and proliferation. Prolyl-hydroxylated HIF- is targeted for proteasomal degradation by the von Hippel-LindauE3 ubiquitin ligase complex. HIF 2OG oxygenases function as O2 sensors because they require O2 for catalysis. Under hypoxia, hydroxylation is inhibited and HIF signaling is activated. In the thick ascending limb of Henle, this activation may be renoprotective for acute ischemic injury.
Read Also: How Does Urine Form In The Kidney
Posttranslational Modifications Of Hif That Control Epo Production
Several posttranslational modifications of HIF-2 have been identified that modulate the systemic EPO response. HIF-2 is acetylated during hypoxia and deacetylated by sirtuin 1, a NAD+-dependent protein deacetylase, which increases HIF-2-dependent EPO synthesis in vitro and in vivo, thereby linking cellular redox and energy state to systemic hypoxia responses . Sirtuin 1-deficient mice produced significantly lower amounts of fetal liver EPO mRNA and during adulthood less renal EPO in response to hypoxia . Interestingly, caloric restriction, which induces sirtuin 1 activity, suppresses EPO production in the liver . Further studies are needed to reconcile these contradictory findings.
Generation Of Conditional Knockout Mice With Hif
To investigate the role of renal HIF-2 in the regulation of serum EPO levels, P3Pro-Cre transgenic mice were crossed to mice homozygous for the conditional Hif-2 allele., Recombination of the conditional Hif-2 allele was highly efficient in kidneys as determined by genomic PCR . This is reflected in an almost complete reduction of Hif-2 exon 2 transcript levels . Although recombination was detectable in liver DNA, we did not find a significant difference in the expression levels of Hif-2 exon 2 between mutant and control livers . As shown in B, P3Pro-Cre is widely expressed in the kidney and in a small subpopulation of hepatocytes. It is not expressed in biliary epithelium and Ito cells . A heterogeneous pattern of limited P3Pro-Cre expression was found in additional tissues, including lung, spleen, and bone marrow . Bone marrow responsiveness to recombinant EPO was normal in P3Pro mutants . Taken together, these studies show that Hif-2 was ablated in most renal cells, whereas loss of Hif-2 in a small percentage of hepatocytes did not significantly affect Hif-2mediated hypoxia responses in the liver.
Also Check: How Much Blood Is Filtered Through The Kidneys Daily
Hypoxic Regulation Of Iron Homeostasis And Bone Marrow Environment
The availability of sufficient amounts of iron is critically important for normal and stress-induced erythropoiesis. Serum iron levels depend on intestinal absorption from the diet, transport capacity in the blood, recycling of iron released from phagocytosed erythrocytes, and the release of iron from other tissue stores, such as the liver. Most of the iron used for normal erythropoiesis is recycled from phagocytosed erythrocytes . When erythropoiesis is stimulated by hypoxia, iron demand in the bone marrow increases. This necessitates increased intestinal iron uptake, an augmentation of serum iron binding capacity and enhanced mobilization of iron from internal stores. Therefore, it is not surprising that some key proteins involved in iron metabolism are oxygen regulated. Bona fide HIF targets involved in maintaining iron homeostasis include transferrin, which transports serum iron in its ferric form to target organs its high-affinity receptor transferrin receptor-1 ceruloplasmin, which oxidizes Fe2+ to Fe3+ and is also important for iron transport the divalent metal transporter-1 , which transports iron into the cytoplasm of cells duodenal cytochrome b , which reduces ferric iron to its ferrous form and heme-oxygenase-1, which is important for the recycling of iron from phagocytosed erythrocytes .
Effects Of Hypoxia On Animal Models Of Obesity
In summary, studies investigating the functions of HIFs in adipocytes and other metabolic diseases revealed the conflicting results due to different experimental conditions . The roles of HIFs in these studies were discovered primarily through the analysis of conditional Hif-knockout mice or through some pharmacological HIF inhibitors. These inconsistent consequences might be also due to the complexity of metabolic regulation with the complicated roles of HIFs that extend further than lipid metabolism. Therefore, the actual functions of HIFs in adipocytes and related metabolic diseases must be carefully interpreted relative to different physiological conditions.
Fig. 3
Hypoxia-regulated lipid metabolism related to obesity. Hypoxia is shown as a promoter or a suppressor of obesity by regulating lipid metabolism. The major changes involved in fatty acid -oxidation, extracellular fatty acid influx, lipolysis, lipogenesis and lipid droplet accumulation under hypoxia for its promoting obesity or anti-obesity effects are summarized
Kidney diseases
Read Also: How To Break Kidney Stones
Immune Response To Hypoxaemia
Immune cells function very well in even extremely hypoxic environments and so their function is not particularly impaired in the face of systemically low oxygen. However, there are various immune phenomena which take place in the hypoxic patient, detailed by Krzywinska & Stockmann. In summary, hypoxia-inducible factors induce a variety of changes in immune cell function, including:
- Increased pro-inflammatory cytokine production
- Stimulation of VEGF
Hepatic Epo Synthesis Is Not Suppressed In P3pro Mutant Mice
Because the main source of EPO changes from the liver to the kidney perinatally, we investigated whether lack of renal Hif-2 had any effect on the developmental switch in tissue Epo expression. PCR analysis of genomic DNA isolated from mutant kidney extracts at different time points after birth indicated comparable recombination efficiency at postnatal day 2 , 10, 20, and 90 . Whereas renal Epo mRNA levels were substantially suppressed at P10, P20, and P90, hepatic Epo in mutant mice did not decline at P10, P20, and P90 compared with littermate control mice, where it became undetectable at P20 and P90 with the use of real-time PCR . Interestingly, renal Epo mRNA levels at P2 did not differ between mutants and controls, suggesting that the regulation of Epo expression at this stage of postnatal development may be Hif-2 independent.
Recommended Reading: Can Coffee Cause Kidney Damage