Kidneys And Acidbase Balance
The kidneys have two very important roles in maintaining the acidbase balance:
The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control pH by the excretion of excess acid or base. The major, homeostatic control point for maintaining a stable pH balance is renal excretion.
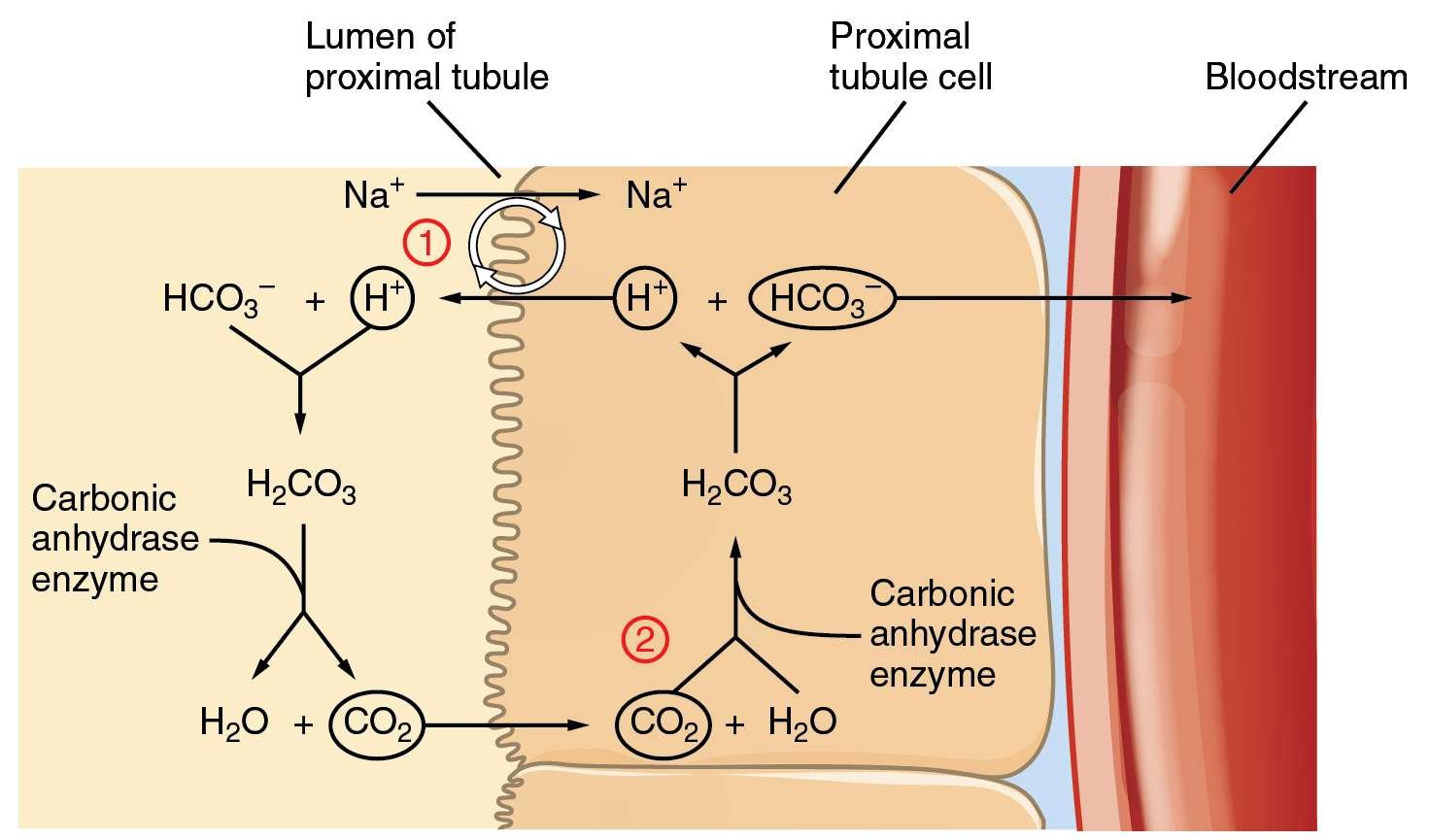
Bicarbonate does not have a transporter, so its reabsorption involves a series of reactions in the tubule lumen and tubular epithelium. In response to acidosis, the tubular cells reabsorb more bicarbonate from the tubular fluid, and the collecting duct cells secrete more hydrogen and generate more bicarbonate, and ammoniagenesis leads to an increase in the formation of the NH3 buffer.
In response to alkalosis, the kidneys may excrete more bicarbonate by decreasing hydrogen ion secretion from the tubular epithelial cells, and lowering the rates of glutamine metabolism and ammonium excretion.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. : Boundless.com. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- This page has no tags.
Intravenous Solutions: Lactated Ringer’s Solution
Another way in which acid-base loads can enter the body is via intravenous solutions. Hospitalized patients receive a variety of intravenous solutions a common one being lactated Ringer’s solution, a mixture of salts that contains lactate at a concentration of 28 mEq/L. The pH is approximately 6.5. However, this is an alkalinizing solution for the same reason described as the fruit juice paradox earlier. Lactate is an organic anion, the conjugate base of lactic acid, and when oxidized to CO2 and water, it takes a hydrogen ion from the body fluids, thereby producing bicarbonate. Lactated Ringer’s should not be confused with a lactic acidosis associated with certain forms of shock. In those situations, the body produces equal numbers of hydrogen ions and lactate and the result is to acidify the body fluids.
A simplified overview of the renal processing of acids and bases is as follows: The acid-base component of highest concentration in the blood is bicarbonate , and even under unusual circumstances, the kidneys have to reabsorb most of the filtered load. This is accomplished primarily in the proximal tubule, thus conserving plasma bicarbonate. The proximal tubule also secretes limited amounts of organic bases or weak organic acids and acid equivalents as previously described in Chapter 5. Then, in the distal nephron , the kidneys secrete either protons or bicarbonate to balance the net input into the body .
Treatment Of Respiratory Acidosis
A doctor should be seen immediately to treat acute respiratory acidosis, as this can be a life threatening condition. Treatment is targeted to the cause.
Bronchodilator medications may be given to correct some forms of airway obstruction. If your blood oxygen level is too low, you may require oxygen. Noninvasive positive pressure ventilation or a breathing machine may be necessary.
To treat chronic respiratory acidosis, the underlying cause needs to be determined in order for proper treatment to take place. The cause could be from an organ deformity, an infection, or some type of inflammation. Each cause may require a different treatment ranging from antibiotics to a breathing machine.
In either case, if you smoke, you will be advised to stop.
Also Check: Constipation Kidney Stones
Anaerobic Metabolism Of Carbohydrate And Fat
The normal oxidative metabolism of carbohydrate and fat is acid-base neutral. Both carbohydrate and triglycerides are oxidized to CO2 and water. Although there are intermediates in the metabolism that are acids or bases, the sum of all the reactions is neutral. However, some conditions lead to production of fixed acids. The anaerobic metabolism of carbohydrate produces a fixed acid . In conditions of poor tissue perfusion or strenuous exercise, this can be a major acidifying factor. The metabolism of triglyceride to -hydroxybutyrate and acetoacetate also adds fixed acid . These processes normally do not add much of an acid load but can add a huge acid load in unusual metabolic conditions .
Metabolic Acidosis And Metabolism
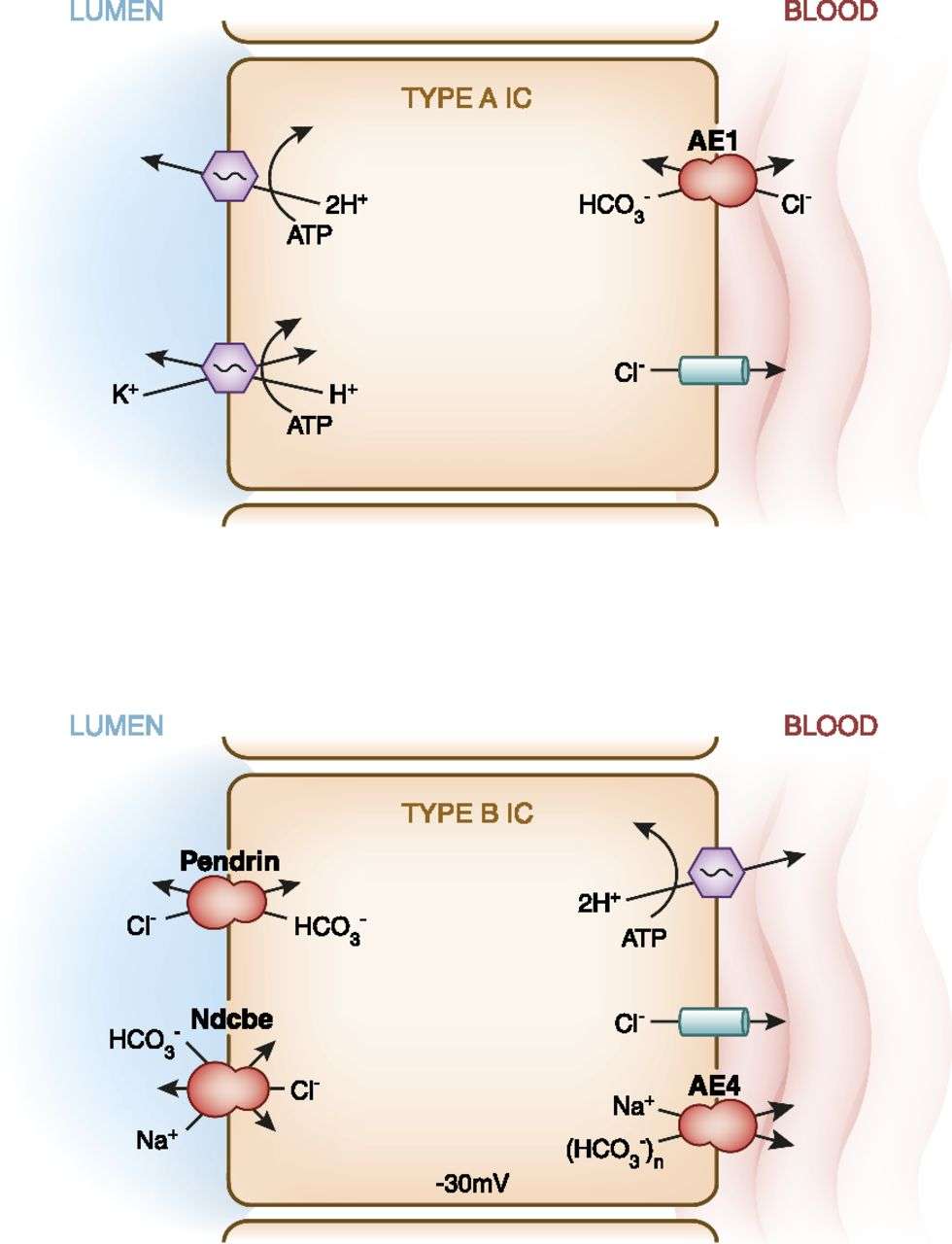
Fig. 2
Summary of main renal metabolic pathways altered between kidney transplant recipients with or without acidosis. Bulk RNA sequencing data using RNA from kidney biopsies of KTRs identified genes altered between patients with or without acidosis, but with comparable eGFR. These genes participate in metabolic activities shown in this figure in black. Red lines show molecular pathways that had genes restored by alkali therapy. Blue arrows show direct biochemical reactions, and blue dashed lines show indirect biochemical reactions. Black arrows show movement of molecules. Data originally published in . TCA cycle tricarboxylic acid cycle , P5P pyridoxal-5-phosphate, GSH glutathione, THF tetrahydrofolate
Two central questions derive from these observations: Does deranged metabolism define trajectories towards faster or slower kidney function decline in chronic kidney disease or does it simply reflect disturbance from other causes? Cippà et al. identified early markers associated with these trajectories in biopsies of patients submitted to renal ischemia and reperfusion because of transplantation . They identified that genes associated with mitochondrial function, senescence, and inflammation were among the most prevalent genes associated with different trajectories. Given the roles of pH in metabolism and mitochondrial function described previously here, is pH a key factor influencing these trajectories, or is it only a sensitive biomarker of kidney damage?
Also Check: Flomax For Kidney Stones In Woman
Metabolism Of Dietary Weak Acids
Fruits and vegetables, particularly citrus fruit, contain many weak acids and the salts of those acids . We all know that citrus juice is acidic, with some fruit juices having a pH below 4.0. Interestingly, metabolism of these acidic substances alkalinizes the blood, sometimes called the fruit juice paradox. The complete oxidation of the protonated form of an organic acid to CO2 and water is acid-base neutral, no different in principle than the oxidation of glucose. However, the complete oxidation of the base form adds bicarbonate to the body, that is, organic anions are precursors of bicarbonate. One can think of metabolizing an organic base anion as taking a hydrogen ion from the body fluids to protonate the anion, thus converting it to a neutral acid, and then oxidizing the acid. The loss of the hydrogen ion, as emphasized above, adds bicarbonate. This loss of hydrogen ions greatly exceeds the number of free hydrogen ions present in the original fruit juice. Although its pH is low, there is far more base than free hydrogen ions. Before oxidation, the mixture is acidic, but on complete oxidation to CO2 and water, the result is addition of base.
Ammonium Elimination By The Distal Nephron
When tubule fluid enters the early distal tubule, the ammonium concentration is about 1.2 mmol/L. Then, something happens, and the ammonium concentration of the distal nephron increases massively. Moreover, it increases even more massively if the organism is experiencing metabolic acidosis, which strongly suggests a regulatory role. Good et al measured a collecting duct ammonium concentration of 33.8 mmol/L, and as high as 73.6 mmol/L in rats with a chronic acidosis.
How does this happen? Nobody actually knows, and the uncertainty is reflected in the broken arrows in the diagram above. The concentration of ammonium in the interstitial fluid of the inner medulla is quite high but clearly that is not high enough to create a concentration gradient and drive the ammonium out by passive diffusion. The ammonium ion itself is positively charged, and to expect that it would cross the membranes on its own is not especially plausible, which suggests that there is some sort of transporter involved. Karim et al document the contribution of RhBG and RhCG proteins which seem to permit the entry of NH3 into the cells, potentially explaining how it moves through the basolateral membrane. For this to happen, NH4+ would need to split into NH3 and H+, get transported into intercalated cells, and then get excreted separately to recombine again in the urine, as depicted in this crude diagram:
Recommended Reading: Does Carbonation Cause Kidney Stones
Hydrogen Ion Excretion On Urinary Bases
We see that the identical transport process of hydrogen ion secretion achieves both reabsorption of bicarbonate , and acid excretion, with addition of new bicarbonate to the blood. At first glance, this seems like a contradiction: How can the same process produce 2 different results? The answer lies in the fate of the hydrogen ion once it is in the lumen. For secreted hydrogen ions that combine with bicarbonate , we are simply replacing filtered bicarbonate that would have left the body. The hydrogen ion is incorporated into water. In contrast, when secreted hydrogen ions combine with a nonbicarbonate base in the lumen of the distal nephron, where little if any bicarbonate remains, the hydrogen ion is excreted and the bicarbonate produced in the cell and transported across the basolateral membrane is new bicarbonate, not a replacement for filtered bicarbonate.
Module : Fluid Electrolyte And Acid
- Identify the most powerful buffer system in the body
- Explain the way in which the respiratory system affects blood pH
Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Acid-balance balance is measured using the pH scale, as shown in. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow pH range, even in the face of perturbations. A buffer is a chemical system that prevents a radical change in fluid pH by dampening the change in hydrogen ion concentrations in the case of excess acid or base. Most commonly, the substance that absorbs the ions is either a weak acid, which takes up hydroxyl ions, or a weak base, which takes up hydrogen ions.
| Table 1. The pH Scale | |
|---|---|
| pH | |
| Battery acid, strong hydrofluoric acid | |
| 1 | Hydrochloric acid secreted by stomach lining |
| 2 | Lemon juice, gastric acid, vinegar |
| 3 | Grapefruit juice, orange juice, soda |
| 4 | |
| Soft drinking water, black coffee | |
| 6 | |
| Great Salt Lake, milk of magnesia | |
| 11 | |
| Liquid drain cleaner |
Also Check: Is Watermelon Good For Kidney
Changes In Ph And Ion Concentration Along The Nephron
Again, its time for one of those huge diagrams of renal tubular pH, with unreadably small writing . The only reason this exists is because in Question 12 from the second paper of 2014, the college asked us to “describe… the changes in urine pH along the nephron”, for 80% of the marks. So, that’s what it would look like if you tried to answer with a diagram:
If this horror has any merit, it is in demonstrating what happens with an increased bicarbonate load, i.e. a metabolic alkalosis. The proximal tubule is the main site of reabsorption, and elsewhere in the tubule the mechanisms of bicarbonate reclamation are relatively weak and lack the capacity to make up for the added bicarbonate delivery to the distal nephron. This means the tubular pH would rapidly approach some sort of maximum value and it would remain there for the length of the tubule.
Koeppen, Bruce M. “The kidney and acid-base regulation.” Advances in physiology education .
McNamara, J., and L. Worthley. “Acid-base balance: part I. Physiology.” Critical Care and Resuscitation 3 : 181-187.
Atherton, John C. “Role of the kidney in acidbase balance.” Anaesthesia & Intensive Care Medicine 16.6 : 275-277.
Weiner, I. David, Jill W. Verlander, and Charles S. Wingo. Core Concepts in the Disorders of Fluid, Electrolytes and Acid-Base Balance. Springer, Boston, MA, 2013. 203-233.
Skelton, Lara A., Walter F. Boron, and Yuehan Zhou. “Acid-base transport by the renal proximal tubule.” Journal of nephrology 23.0 16 : S4.
The Role Of The Kidney In Acid
The kidneys have two main ways to maintain acid-base balance – their cells reabsorb bicarbonate HCO3 from the urine back to the blood and they secrete hydrogen H+ ions into the urine.
Our kidneys filter blood continuously by distributing the blood that comes into the kidney to millions of tiny functional units called .
Each is made up of the glomerulus, or a tiny clump of , where blood filtration begins.
When blood passes through a glomerulus, about one-fifth of the plasma leaves the glomerular capillaries and goes into the renal tubule.
Reabsorption of the good stuff—water and electrolytes—and leaving behind the bad stuff—waste products and acid— is the job of the the .
The renal tubule is a structure with several segments: the , the U- shaped with a thin descending and a thick ascending limb, and the , which winds and twists back up again, before emptying into the collecting duct, which collects the final urine.
Each of these tubules is lined by brush border cells which have two surfaces.
One is the apical surface that faces the tubular lumen and is lined with microvilli, which are tiny little projections that increase the cells surface area to help with solute reabsorption.
The other is the basolateral surface, which faces the peritubular capillaries, which run alongside the nephron.
So with , as the filtrate leaves the glomerulus, it first goes through the proximal convoluted tubule.
You May Like: Matcha Kidney Stones
Other Factors Which Affect Renal Acid
There a few other peripheral matters to attend do, with regards to renal acid-base handling. As all must surely have noticed by now, the movements of sodium and potassium are key to renal acid-base balance management. And as sodium handling and potassium handling are tightly regulated by various neurohormonal actors, it stands to reason that these actors will have some indirect effect on acid-base handling as well.
Pulmonary Role In The Acid
Various Buffer systems and their role in the acid-base system:
| Buffer system | ||
|
|
||
|
HCO3 / H2CO3 |
|
|
| Protein / hydrogenated protein | Plasma proteins |
|
| HPO4 / H2PO4 |
Recommended Reading: Can A Kidney Infection Cause Diarrhea
Functions Ofthe Buffer System:
How Do We Maintain Water Balance In The Body
Thirst is one of the most important mechanisms to maintain water balance. When the body needs water, nerve centers deep within the brain are stimulated, resulting in the sensation of thirst. The sensation becomes stronger as the bodys need for water increases, motivating a person to drink the needed fluids.
Read Also: Does Red Wine Cause Kidney Stones
What Is The Role Of Urinary Ammonium Excretion
There are different views on the true role of NH4+ excretion in urine. How can the renal excretion of ammonium which has a pK of 9.2 represent H+ excretion from the body?
One school says the production of ammonium from glutamine in the tubule cells results in production of alpha-ketoglutarate which is then metabolised in the tubule cell to new bicarbonate which is returned to the blood. The net effect is the return of one bicarbonate for each ammonium excreted in the urine. By this analysis, the excretion of ammonium is equivalent to the excretion of acid from the body as one plasma H+ would be neutralised by one renal bicarbonate ion for each ammonium excreted. Thus an increase in ammonium excretion as occurs in metabolic acidosis is an appropriate response to excrete more acid.
The other school says this is not correct. The argument is that metabolism of alpha-ketogluarate in theproximal tubule cells to produce this new HCO3- merely represents regeneration of the HCO3 that was neutralised by the H+ produced when alpha-ketoglutarate was metabolised to glutamate in the liver originally so there can be no direct effect on net H+ excretion. The key to understanding is said to lie in considering the role of the liver. Consider the following:
- How to get rid of 1,000mmol/day of alkali?
- How to get rid of 1,000mmol/day of the highly toxic ammonium?
2 NH4+ + 2 HCO3- => urea + CO2 + 3 H2O
The body has two ways in which it can remove NH4+:
Other points are:
Renal Control Of Acid Base Balance
Read Also: Can Stress Cause Kidney Infection