Causes And Diagnostic Considerations
Metabolic acidosis is typically classified according to whether the anion gap is normal or high. Non-AG metabolic acidosis is also characterized by hyperchloremia and is sometimes referred to as hyperchloremic acidosis. Calculation of the AG is thus helpful in the differential diagnosis of metabolic acidosis.
Normal anion gap metabolic acidosis
Hyperchloremic or non-AG metabolic acidosis occurs principally when HCO3- is lost from either the GI tract or the kidneys or because of a renal acidification defect. Some of the mechanisms that result in a non-AG metabolic acidosis are the following:
-
Addition of HCl to body fluids: H+ buffers HCO3- and the added Cl- results in a normal AG.
-
Loss of HCO3- from the kidneys or the GI tract: The kidneys reabsorb sodium chloride to maintain volume.
-
Rapid volume expansion with normal saline: This results in an increase in the chloride load that exceeds the renal capacity to generate equal amounts of HCO3-.
Causes of non-AG metabolic acidosis can be remembered with the mnemonic ACCRUED .
The conditions that may cause a non-AG metabolic acidosis are as follows:
-
GI loss of HCO3- – Diarrhea
-
Enterocutaneous fistula – Enteric diversion of urine , pancreas transplantation with bladder drainage
-
Renal loss of HCO3- – Proximal RTA , carbonic anhydrase inhibitor therapy
-
Failure of renal H+ secretion – Distal RTA , hyperkalemic RTA , renal failure
-
Acid infusion – Ammonium chloride, hyperalimentation
-
Other – Rapid volume expansion with normal saline
Potassium And Renal Acid Secretion
Renal acid secretion is influenced by serum K+ and may result from the transcellular shift of K+ when intracellular K+ is exchanged for extracellular H+ or vice versa. In hypokalemia, an intracellular acidosis can develop in hyperkalemia, an intracellular alkalosis can develop. HCO3- reabsorption is increased secondary to relative intracellular acidosis. The increase in intracellular H+ concentration promotes the activity of the apical Na+/H+ exchanger.
Renal production of NH3 is increased in hypokalemia, resulting in an increase in renal acid excretion. The increase in NH3 production by the kidneys may be significant enough to precipitate hepatic encephalopathy in patients who have advanced liver disease. Correcting the hypokalemia can reverse this process.
Patients with hypokalemia may have relatively alkaline urine because hypokalemia increases renal ammoniagenesis. Excess NH3 then binds more H+ in the lumen of the distal nephron and urine pH increases, which may suggest RTA as an etiology for non-AG acidosis. However, these conditions can be distinguished by measuring urine AG, which will be negative in patients who have normal NH4+ excretion and positive in patients with RTA. The most common cause for hypokalemia and metabolic acidosis is GI loss . Other less common etiologies include renal loss of potassium secondary to RTA or salt-wasting nephropathy. The urine pH, the urine AG, and the urinary K+ concentration can distinguish these conditions.
Fluid Movement Between Compartments
Hydrostatic pressure, the force exerted by a fluid against a wall, causes movement of fluid between compartments. The hydrostatic pressure of blood is the pressure exerted by blood against the walls of the blood vessels by the pumping action of the heart. In capillaries, hydrostatic pressure is higher than the opposing colloid osmotic pressure in blooda constant pressure primarily produced by circulating albuminat the arteriolar end of the capillary . This pressure forces plasma and nutrients out of the capillaries and into surrounding tissues. Fluid and the cellular wastes in the tissues enter the capillaries at the venule end, where the hydrostatic pressure is less than the osmotic pressure in the vessel. Filtration pressure squeezes fluid from the plasma in the blood to the IF surrounding the tissue cells. The surplus fluid in the interstitial space that is not returned directly back to the capillaries is drained from tissues by the lymphatic system, and then re-enters the vascular system at the subclavian veins.
Figure 26.7 Capillary Exchange Net filtration occurs near the arterial end of the capillary since capillary hydrostatic pressure is greater than blood colloidal osmotic pressure . There is no net movement of fluid near the midpoint of the capillary since CHP = BCOP. Net reabsorption occurs near the venous end of the capillary since BCOP is greater than CHP.
Also Check: Grapes And Kidney Stones
Pathogenesis Of Essential Hypertension
These considerations may be relevant to the pathogenesis of so-called âessentialâ hypertension, in which a specific underlying cause for increased blood pressure cannot be defined in identifiable pathology in any organ system. This pattern, which would match the initial hypertensive history of our patient Mr Schneider, is associated with a family history of hypertension and the development of an increased blood pressure in the affected subject during the third to fifth decade of life. While the precise pathogenesis of this condition has not been definitively established , one plausible scenario is shown in Fig. 10.2.
Fig. 10.2. Chronic volume expansion and hypertension: a model for essential hypertension? CNS, central nervous system ECF, extracellular fluid TPR, total peripheral resistance.
While speculative, the schema described above does accord with much of the available data about renal and circulatory changes observed during the generation and maintenance of hypertension. It also gives a framework for considering other factors known to predispose towards hypertension, whether or not there is another pathologically definable cause. A list of such risk factors is provided in Box 10.1.
-
Use of non-steroidal anti-inflammatory drugs
-
Steroid therapy
-
Excessive use of topical or systemic vasoconstrictor medications
ECF volume expansion also has an important role in the genesis of a number of forms of secondary hypertension these are discussed later in this chapter.
Calculate The Anion Gap
For a patient with metabolic acidosis it can be useful to calculate the anion gap because this can give some indication of the underlying cause of the acid-base imbalance.
The anion gap is the difference between the measured positively charged cations and the negatively charged anions . The following equation can be used to estimate the anion gap:
An increased anion gap indicates excess acid from the anions that are unmeasured . It is also worth noting that a drop in a patients albumin lowers the anion gap. A deranged phosphate level can also affect the anion gap, but to a lesser extent.
Box 3: Classification
Don’t Miss: Grapes For Kidney Stones
How Buffers Work: A Quantitative View
The kidneys and the lungs work together to help maintain a blood pH of 7.4 by affecting the components of the buffers in the blood. To understand how these organs help control the pH of the blood, we must first discuss how buffers work in solution.
Acid-base buffers confer resistance to a change in the pH of a solution when hydrogen ions or hydroxide ions are added or removed. An acid-base buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid . A buffer works because it contains a substantial amount of a weak acid and a weak base at equilibrium with each other. When protons are added to the buffer, some of the base component of the buffer will react with the protons and turn into the conjugate acid and thus neutralizing most of the protons added. When hydroxide ions are added , some of the weak-acid component of the buffer will dissociate and turn into the conjugate base thus replenishing most of the protons removed. Hence, adding a small amount of acid or base to a buffer solution merely changes the ratio of the conjugate acid and conjugate base in an acid-base equilibrium. Thus, the effect on the pH of the solution is small, within certain limitations of the amount of H+or OH- added or removed. Some fundamental acid-base concepts are summarized in the box below.
Recap Of Fundamental Acid
Dissociation of Water and the p-Scale
Water molecules dissociate to form H+ and OH- ions:
Note that and are the concentrations of the weak acid and its conjugate base at equilibrium. Since the dissociation of a weak acid is typically very small, we can assume that and are similar to the initial concentrations, i and i. Equation 8 is known as the Henderson-Hasselbalch Equation. This equation shows that the pH of a buffer solution is very close to the pKa value of the weak acid making the buffer. This is because the logarithm term will be small unless the concentrations of A- and HA differ by several orders of magnitude.
For a basic buffer consisting of a weak base and its conjugate acid, one can begin with Eq. and follow similar derivation steps to obtain:
Recommended Reading: What Laxative Is Safe For Kidneys
Specific Causes Of Hyperchloremic Or Non
Loss of HCO3- via the GI tract
The secretions of the GI tract, with the exception of the stomach, are relatively alkaline, with high concentrations of base . Significant loss of lower GI secretions results in metabolic acidosis, especially when the kidneys are unable to adapt to the loss by increasing net renal acid excretion.
Such losses can occur in diarrheal states, fistula with drainage from the pancreas or the lower GI tract, and sometimes vomiting if it occurs as a result of intestinal obstruction. When pancreatic transplantation is performed, the pancreatic duct is sometimes diverted into the recipient bladder, from where exocrine pancreatic secretions are lost in the final urine. Significant loss also occurs in patients who abuse laxatives, which should be suspected when the etiology for non-AG metabolic acidosis is not clear.
Urine pH will be less than 5.3, with a negative urine AG reflecting normal urine acidification and increased NH4+ excretion. However, if distal Na+ delivery is limited because of volume depletion, the urine pH cannot be lowered maximally.
Replacing the lost HCO3- on a daily basis can treat this form of metabolic acidosis.
Distal RTA
Several different mechanisms are implicated in the development of distal RTA. These include a defect in 1 of the 2 proton pumps, H+ ATPase or K+ -H+ ATPase, that can be acquired or congenital. This may lead to loss of function or a reduction in the rate of H+ secretion .
-
Primary – Genetic or sporadic
What Is The Treatment Of Metabolic Acidosis
Treatment of acute metabolic acidosis by alkali therapy is usually indicated to raise and maintain the plasma pH to greater than 7.20. In the following two circumstances this is particularly important. When the serum pH is below 7.20, a continued fall in the serum HCO3- level may result in a significant drop in pH.
Don’t Miss: Seltzer Water And Kidney Stones
When The Ph Of The Extracellular Fluid Drops The
- School
- 50%1 out of 2 people found this document helpful
This preview shows page 6 – 8 out of 20 pages.
University of the Fraser Valley
BIO BIO123
Wayne State University BIO 2870
ch27
Lone Star College, Tomball BIOL 2402
Random Q Chapter 19-20-21.pdf
Lone Star College, Tomball BIOL 2402
Random Q, Chap 16-18.pdf
University of the Fraser Valley BIO BIO123
17.docx
How Do You Know If You Have Metabolic Respiratory Acidosis Alkalosis
You May Like: Kidney Pain Std
Hypokalemia: Lessons From The Salt
Genetic studies of inborn errors resulting in renal salt wasting have provided novel insights into the pathophysiology of renal electrolyte handling. Additional studies on engineered mice and cell culture have further highlighted the function of specific transport proteins and the molecular pathways regulating their activity.
Bartter and Gitelman syndromes are genetic disorders leading to hypokalemic metabolic alkalosis due to renal loss, with a compensatory activation of the RAAS axis. Genetic studies have shed light onto the molecular basis underlying these disorders that have long been considered two forms of the same disease .
Table 1
For additional information:
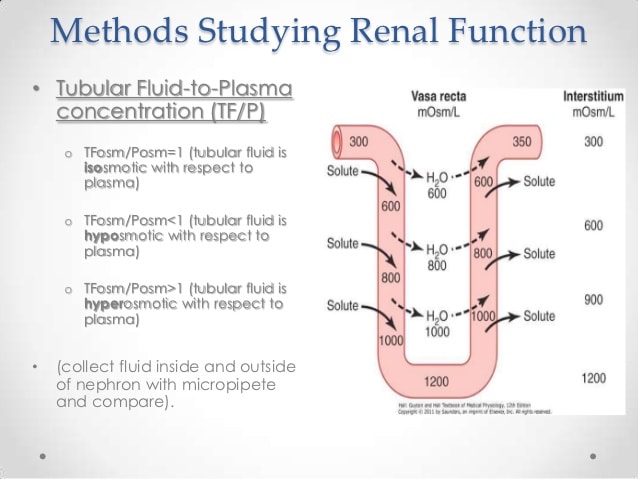
Fluid Compartments In An Average 70
|
Total body water = 70 kg × 0.60 = 42 L . |
The major intracellular cation is potassium. The major extracellular cation is sodium. Concentrations of intracellular and extracellular cations are as follows:
-
Intracellular potassium concentration averages 140 mEq/L .
-
Extracellular potassium concentration is 3.5 to 5 mEq/L .
-
Intracellular sodium concentration is 12 mEq/L .
-
Extracellular sodium concentration averages 140 mEq/L .
Read Also: Is Cranberry Juice Good For Your Liver And Kidneys
How Does Exercise Affect The Body
Many people today are interested in exercise as a way of improving their health and physical abilities. When we exercise, our heart rate, systolic blood pressure, and cardiac output all increase. Blood flow to the heart, the muscles, and the skin increase. The body’s metabolism becomes more active, producing CO2 and H+ in the muscles. We breathe faster and deeper to supply the oxygen required by this increased metabolism. With strenuous exercise, our body’s metabolism exceeds the oxygen supply and begins to use alternate biochemical processes that do not require oxygen. These processes generate lactic acid, which enters the blood stream. As we develop a long-term habit of exercise, our cardiac output and lung capacity increase, even when we are at rest, so that we can exercise longer and harder than before. Over time, the amount of muscle in the body increases, and fat is burned as its energy is needed to help fuel the body’s increased metabolism.
How Is Ecf Volume Regulated
If ECF volume is to remain constant, the amount of sodium ingested must be matched by the amount of sodium excreted by the kidneys. In the example above, expansion of the ECF volume must somehow be sensed. What is sensed is not ECF volume, but rather a portion of ECF called effective arterial volume. Effective arterial volume is that portion of the ECF that is in the arterial tree and effectively perfusing tissues. An increase in effective arterial volume is sensed by baroreceptors in the aortic arch, carotid sinus, central veins, cardiac chambers, and afferent arterioles. In addition, an increase in effective arterial volume leads to an increase in renal tubular flow, which is sensed by the macula densa. Signals are then sent to the kidneys, which lead to diminished sodium reabsorption by the renal tubules and increased sodium excretion by the kidneys. In the example above, 150 mmol of sodium will be excreted by the kidneys, returning ECF volume to normal.
Michael Allon, in, 2014
Recommended Reading: Is Honey Good For Kidney
Renal Regulation Of Acid
The renal regulation of the bodys acid-base balance addresses the metabolic component of the buffering system. Whereas the respiratory system controls the blood levels of carbonic acid by controlling the exhalation of CO2, the renal system controls the blood levels of bicarbonate. A decrease of blood bicarbonate can result from the inhibition of carbonic anhydrase by certain diuretics or from excessive bicarbonate loss due to diarrhea. Blood bicarbonate levels are also typically lower in people who have Addisons disease , in which aldosterone levels are reduced, and in people who have renal damage, such as chronic nephritis. Finally, low bicarbonate blood levels can result from elevated levels of ketones , which bind bicarbonate in the filtrate and prevent its conservation.
Bicarbonate ions, HCO3-, found in the filtrate, are essential to the bicarbonate buffer system, yet the cells of the tubule are not permeable to bicarbonate ions. The steps involved in supplying bicarbonate ions to the system are seen in Figure 26.17 and are summarized below:
Figure 26.17 Conservation of Bicarbonate in the Kidney Tubular cells are not permeable to bicarbonate thus, bicarbonate is conserved rather than reabsorbed. Steps 1 and 2 of bicarbonate conservation are indicated.
Regulation Of Calcium And Phosphate
Calcium and phosphate are both regulated through the actions of three hormones: parathyroid hormone , dihydroxyvitamin D , and calcitonin. All three are released or synthesized in response to the blood levels of calcium.
PTH is released from the parathyroid gland in response to a decrease in the concentration of blood calcium. The hormone activates osteoclasts to break down bone matrix and release inorganic calcium-phosphate salts. PTH also increases the gastrointestinal absorption of dietary calcium by converting vitamin D into dihydroxyvitamin D , an active form of vitamin D that intestinal epithelial cells require to absorb calcium.
PTH raises blood calcium levels by inhibiting the loss of calcium through the kidneys. PTH also increases the loss of phosphate through the kidneys.
Calcitonin is released from the thyroid gland in response to elevated blood levels of calcium. The hormone increases the activity of osteoblasts, which remove calcium from the blood and incorporate calcium into the bony matrix.
Read Also: Bleeding Kidney Treatment
Large Volume Or Rapid Transfusion Into Neonates And Small Children
Extracellular fluid in cellular blood products contain anticoagulant , dextrose, and, through storage, potassium, and lactate. RBCs rely on the membrane sodiumâpotassium pump to maintain higher intracellular potassium than the extracellular environment, and these pumps are energy adenosine triphosphate dependent and temperature sensitive. During refrigeration of the RBC unit, potassium leaks out of the RBC. Irradiation of RBCs is known to cause injury to the RBC membrane, leading to increased permeability to potassium, shortening the storage time to maximum of 28 days. If longer stored products are infused rapidly or in large volume to a neonate or pediatric patient, there is risk of hyperkalemia-induced cardiac arrhythmia, hyperglycemia, and citrate toxicity . Cases of acute cardiac arrhythmia secondary to hyperkalemia leading to death have been reported in pediatric patients undergoing cardiac surgery, especially during rapid infusion through a central line. Washing or volume reduction has been shown to decrease the risk of hyperkalemia.
Jean-Pierre Guignard, Endre Sulyok, in, 2012
Water Intake And Excretion
The average daily fluid intake is about 2.5 L. The amount needed to replace losses from the urine and other sources is about 1 to 1.5 L/day in healthy adults. However, on a short-term basis, an average young adult with normal kidney function may ingest as little as 200 mL of water each day to excrete the nitrogenous and other wastes generated by cellular metabolism. More is needed in people with any loss of renal concentrating capacity. Renal concentrating capacity is lost in
-
Older people
-
People who ingest ethanol, phenytoin, lithium, demeclocycline, or amphotericin B
-
People with osmotic diuresis
Other obligatory water losses are mostly insensible losses from the lungs and skin, averaging about 0.4 to 0.5 mL/kg/hour or about 650 to 850 mL/day in a 70-kg adult. With fever, another 50 to 75 mL/day may be lost for each degree Celsius of temperature elevation above normal. Gastrointestinal losses are usually negligible, except when marked vomiting, diarrhea, or both occur. Sweat losses can be significant during environmental heat exposure or excessive exercise.
Water intake is regulated by thirst. Thirst is triggered by receptors in the anterolateral hypothalamus that respond to increased plasma osmolality or decreased body fluid volume. Rarely hypothalamic dysfunction decreases the capacity for thirst.
-
Increased plasma osmolality
-
Stress
Don’t Miss: Fluid Buildup Around Kidney