Icipant Recruitment And Ethics
Participants were recruited on a voluntary basis to undergo serial arterial blood gas measurements during a research expedition to Everest base camp in the Nepal Himalaya. Inclusion criteria included adult participants over 18 years of age who planned to trek the entire journey and those willing to provide free, verbal and written informed and ongoing consent. We pre-screened and recruited 20 healthy participants for inclusion with no known cardiovascular, respiratory, renal or metabolic disorders. One male participant was taking anti-hypertensive medication. This study abided by the Canadian Government Tri-Council policy on research ethics with human participants and conformed with the standards set by the latest revision of the , except for registration in a database. Ethical approval was received in advance through the Mount Royal University Human Research Ethics Board , University of Alberta Health Research Ethics Board Biomedical Panel and was harmonized with the Nepal Health Research Council . Although this study was taking place in the context of a large research expedition to high altitude, the specific study design, research question and data collection were planned a priori.
How Is It Treated
Bicarbonate: We all need bicarbonate in our blood. Low bicarbonate levels in the blood are a sign of metabolic acidosis. It is an alkali , the opposite of acid, and can balance acid. It keeps our blood from becoming too acidic. Healthy kidneys help keep your bicarbonate levels in balance. Low bicarbonate levels can also cause your kidney disease to get worse. A small group of studies have shown that treatment with sodium bicarbonate or sodium citrate pills can help keep kidney disease from getting worse. However, you should not take sodium bicarbonate or sodium citrate pills unless your healthcare provider recommends it.
Diet: Increasing fruit and vegetable intake may decrease acid load in the body. This is because fruits and vegetables produce alkali, whereas foods such as meats, eggs, cheese, and cereal grains cause the body to make acid. Your kidney dietitian can show you how to safely increase the right type and amounts of fruits and vegetables in your diet based on your stage of kidney disease.
What Is An Acid What Is A Base And What Is Ph
An acid is a substance which releaseshydrogen ions on dissociation in solution.
For example: Hydrochloric acid dissociates to hydrogenions and chloride ions
Carbonic acid dissociates tohydrogen ions and bicarbonate ions
H2CO3 H+ +HCO3
We distinguish between strong acids like hydrochloric acid andweak acids like carbonic acid. The difference is that strong acidsdissociate more than weak acids. Consequently the hydrogen ionconcentration of a strong acid is much higher than that of a weakacid.
A base is a substance which in solution acceptshydrogen ions.
For example, the base bicarbonate accepts hydrogen ions to form carbonic acid:
HCO3 + H+H2CO3
pH is a scale of 0-14 of acidity and alkalinity. Pure water hasa pH of 7 and is neutral . pH above 7is alkaline and below 7 acidic. Thus the pH of blood isslightly alkaline although in clinical medicine the term alkalosisis, perhaps confusingly, reserved for blood pH greater than 7.45and the term acidosis is reserved for blood pH less than 7.35.
pH is a measure of hydrogen ion concentration .The two are related according to the followingequation:
pH = – log10
where is the concentration of hydrogen ions inmoles per liter
From this equation
pH 7.4 = H+ concentration of 40 nmol/LpH 7.0 = H+ concentration of 100 nmol/LpH 6.0 = H+ concentration of 1000 nmol/L
It is clear that:
Don’t Miss: Grapes For Kidney Stones
Renal Control Of Plasma Hco3
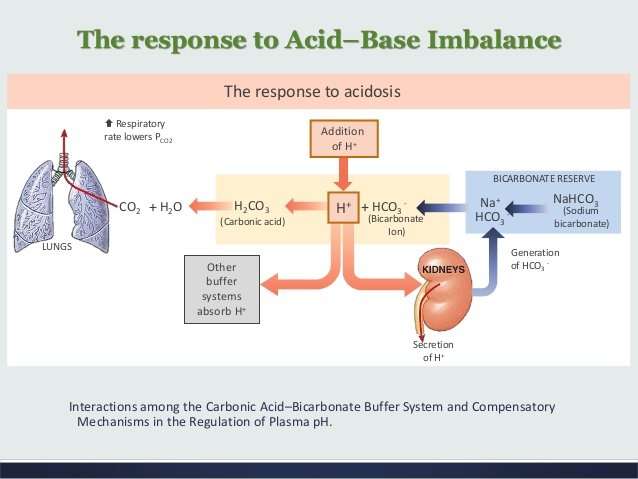
The kidneys have the predominant role of regulating the systemic HCO3 concentration and hence, the metabolic component of acid-base balance. This function of the kidneys has two components: reabsorption of virtually all of the filtered HCO3 and production of new HCO3 to replace that consumed by normal or pathologic acids. This production or generation of new HCO3 is done by net acid excretion. In other words, the kidneys make new HCO3 by excreting acid.
Because HCO3 is freely filtered at the glomerulus, approximately 4.5 mol HCO3 is normally filtered per day . Virtually all of this filtered HCO3 is reabsorbed, with the urine normally essentially free of HCO3. Seventy to eighty percent of this filtered HCO3 is reabsorbed in the proximal tubule the rest is reabsorbed along more distal segments of the nephron .
Relative HCO3transport along the nephron. Most of the filtered HCO3 is reabsorbed in the proximal tubule. Virtually no HCO3 remains in the final urine. CCD, cortical collecting duct DT, distal convoluted tubule IMCD, inner medullary collecting duct TAL, thick ascending limb.
Relative urinary titratable acid and ammonia in adults on a control or acid loading diet . Reference .
The Hypoxic Ventilatory Response
Among the first responses elicited following exposure to acute hypoxic conditions is an increase in resting ventilation, known as the HVR . The HVR partially corrects levels for a given inspired however, a concomitant decrease in the partial pressure of arterial CO2 results, which blunts both central and peripheral respiratory chemoreceptor activation and subsequent ventilatory drive. Thus, if the HVR is to be effective in increasing and in the context of high altitude, the imbalance associated with the blunting effects of hypocapnia must be countered, which involves increasing the HVR sensitivity through carotid body plasticity . Although this increase in the HVR improves oxygenation, further hypocapnia and acid-base imbalance results. Because of the opposing effects ventilation has on and , and the concomitant respiratory alkalosis, a sole adjustment of the HVR would not be an adequate response mechanism in maintaining acid-base homeostasis to chronic hypoxia renal response mechanisms are also required.
You May Like: Is Honey Good For Kidney
Biochemical Features Of Respiratory Alkalosis
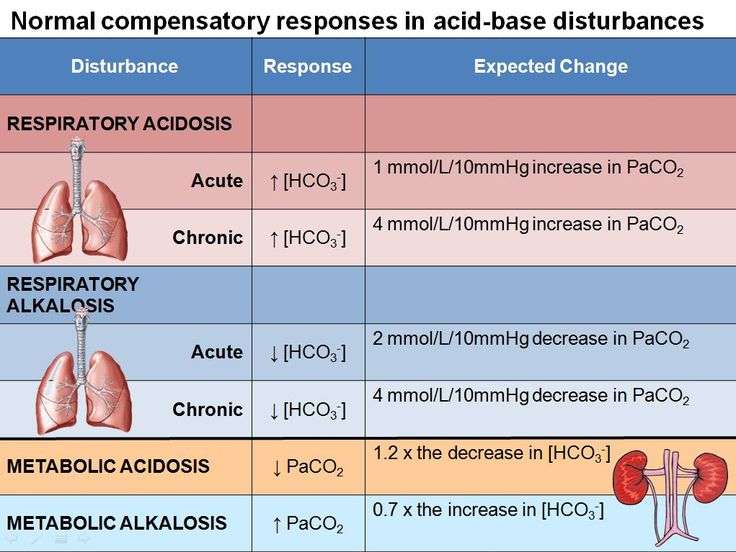
The cardinal feature of an acute respiratory alkalosis is a decrease in arterial PCO2, a decrease in hydrogen ion concentration and a small decrease in bicarbonate concentration, though not to less than about 18 mmol/L. In a chronic respiratory alkalosis, renal compensation may result in arterial hydrogen ion concentration being only marginally decreased, while the bicarbonate concentration falls further, but not to less than about 12 mmol/L. The finding of bicarbonate concentrations less than these values suggests the additional presence of a non-respiratory acidosis.
If the stimulus to hyperventilation is hypoxaemia, arterial hydrogen ion concentration may be affected predominantly by a resulting non-respiratory acidosis. The interpretation of measured acidbase parameters in mixed disorders of hydrogen ion homoeostasis is discussed in a later section.
Robert G. Carroll PhD, in, 2007
Fetoplacental Elimination Of Metabolic Acid Load
Fetal respiratory and renal compensation in response to changes in fetal pH is limited by the level of maturity and the surrounding maternal environment. However, although the placentomaternal unit performs most compensatory functions,4 the fetal kidneys have some, although limited, ability to contribute to the maintenance of fetal acid-base balance.
The most frequent cause of fetal metabolic acidosis is fetal hypoxemia owing to abnormalities of uteroplacental function or blood flow, or both. Primary maternal hypoxemia or maternal metabolic acidosis secondary to maternal diabetes mellitus, sepsis, or renal tubular abnormalities is an unusual cause of fetal metabolic acidosis.
The pregnant woman, at least in late gestation, maintains a somewhat more alkaline plasma environment compared with that of nonpregnant controls. This pattern of acid-base regulation in pregnant women is present during both resting and after maximal exertion and may serve as a protective mechanism from sudden decreases in fetal pH. Maintenance of the less acidic environment during pregnancy appears to be achieved through reduced plasma carbon dioxide and weak acid concentrations.4,11
Devin Eckstein, Howard E. Corey, in, 2019
Agostino Pierro, … Evelyn Ong, in, 2006
Recommended Reading: Va Rating For Chronic Kidney Disease Stage 3
The Diagnostic Approach To Identifying Acid
The usual diagnostic approach to an acid-base disorder begins with a complete history and physical examination. Clues in the history include: understanding the quantity, contents, and source of fluid losses or gains from the body ingested substances and certain diseases known to be associated with acid-base disorders. Examples include vomiting , diarrhea , chronic obstructive pulmonary disease , pneumonia , and so on. Laboratory tests are usually performed including a basic metabolic profile with electrolytes: sodium, potassium, chloride, bicarbonate, blood urea nitrogen, and creatinine. The bicarbonate concentration alone does not prove a metabolic disturbance because there are two other variables in equilibrium with bicarbonate: carbon dioxide and the hydrogen ion concentration. A low plasma bicarbonate is consistent with either metabolic acidosis or respiratory alkalosis. Respiratory alkalosis can be mistaken for renal tubular acidosis if only the plasma bicarbonate is measured. The level will be low, and the urine pH elevated, in both renal acidosis and respiratory alkalosis a blood gas analysis of pH will distinguish the two disturbances.
Table 2
Causes of anion gap metabolic acidosis
Table 3
Causes of hyperchloremic acidosis
Neonatal Renal Compensatory Mechanism
As mentioned earlier, by regulating bicarbonate and acid secretion in response to changes in extracellular pH, renal compensation is the ultimate mechanism to adjust the H+ content of the body. Although full activation of this system usually requires 2 to 3 days, alterations in renal acidification may be seen as early as a few hours after the development of acid-base disturbance.
The renal compensatory mechanisms of the neonate are immature, resulting in a developmentally regulated decreased ability to maintain acid-base balance.19,20 Both renal microhemodynamic and tubular epithelial factors play a role in the limited renal compensatory capacity of the newborn.
Medications used in the treatment of critically ill neonates may also affect proximal tubular bicarbonate reabsorption. For example, dopamine administration may potentially decrease the low bicarbonate threshold of the neonate30 because the drug inhibits activity of the proximal tubular Na+/H+ antiporter.31 Carbonic anhydrase inhibitors also decrease proximal tubular bicarbonate reabsorption by limiting bicarbonate formation and H+ ion availability for the Na+/H+ countertransporter. Furosemide, acting on several transport proteins along the nephron, directly increases urinary excretion of titratable acids and ammonium.32
Also Check: Constipation Kidney Stones
Treatment Of Respiratory Acidosis
A doctor should be seen immediately to treat acute respiratory acidosis, as this can be a life threatening condition. Treatment is targeted to the cause.
Bronchodilator medications may be given to correct some forms of airway obstruction. If your blood oxygen level is too low, you may require oxygen. Noninvasive positive pressure ventilation or a breathing machine may be necessary.
To treat chronic respiratory acidosis, the underlying cause needs to be determined in order for proper treatment to take place. The cause could be from an organ deformity, an infection, or some type of inflammation. Each cause may require a different treatment ranging from antibiotics to a breathing machine.
In either case, if you smoke, you will be advised to stop.
Module : Fluid Electrolyte And Acid
- Identify the three blood variables considered when making a diagnosis of acidosis or alkalosis
- Identify the source of compensation for blood pH problems of a respiratory origin
- Identify the source of compensation for blood pH problems of a metabolic/renal origin
Normal arterial blood pH is restricted to a very narrow range of 7.35 to 7.45. A person who has a blood pH below 7.35 is considered to be in acidosis , and a continuous blood pH below 7.0 can be fatal. Acidosis has several symptoms, including headache and confusion, and the individual can become lethargic and easily fatigued. A person who has a blood pH above 7.45 is considered to be in alkalosis, and a pH above 7.8 is fatal. Some symptoms of alkalosis include cognitive impairment , tingling or numbness in the extremities, muscle twitching and spasm, and nausea and vomiting. Both acidosis and alkalosis can be caused by either metabolic or respiratory disorders.
As discussed earlier in this chapter, the concentration of carbonic acid in the blood is dependent on the level of CO2 in the body and the amount of CO2 gas exhaled through the lungs. Thus, the respiratory contribution to acid-base balance is usually discussed in terms of CO2 . Remember that a molecule of carbonic acid is lost for every molecule of CO2 exhaled, and a molecule of carbonic acid is formed for every molecule of CO2 retained.
Don’t Miss: Can Seltzer Water Cause Kidney Stones
Acute And Chronic Respiratory Acidosis In Chronic Lung Diseases
Metabolism in tissues rapidly generates a large quantity of volatile acid and nonvolatile acids , with a normal CO2 production per day of about 13,000 mmol. In a healthy condition, the lungs excrete the volatile fraction through ventilation, and acid accumulation does not occur. However, a failure of alveolar ventilation reduces pulmonary CO2 elimination, quickly raises Paco2, and finally leads to respiratory acidosis. For instance, if pulmonary ventilation were to cease for 20 minutes in a human, Paco2 would rise to 110 mm Hg and arterial pH would fall to 7.03. In contrast, if renal function were to cease for a similar period, no changes in arterial pH would occur.1 The reference range for Paco2 is 35 to 45 mm Hg . However, Paco2 values between 80 and 100 mm Hg have been reported in patients with COPD breathing air at sea level.18 Higher Paco2 values in patients breathing room air are incompatible with life, because the corresponding Pao2 values would be 20 to 40 mm Hg.1
Renal Compensation for Respiratory Acidosis
Timur Azhibekov, … Istvan Seri, in, 2017
Two Approaches To Understanding Acid
Since only a single blood pH exists at a given time , the must be in equilibrium with many buffer substances that can take up or release hydrogen according to individual rate constants, which are related to a dissociation constant known as the pK. As blood pH changes, the contribution of each buffer-pair to take up hydrogen changes, making the bookkeeping for the very complex. In other words, what is the fate of those hydrogen ions as pH changes from normal? Thus, it is problematic to choose a single buffer system to explain acid-base phenomena. Yet, the isohydric principle is the basis of the traditional approach using bicarbonate and pCO2 to understand acid-base balance .
CO2 + H2O H2CO3 HCO3- + H+
pH = pK + log /0.03
HCO3- deficit = × 0.5 × body weight
Another consequence of the isohydric principle is that in vomiting-induced losses of hydrochloric acid, the amount of hydrogen ion that is lost is equal to the chloride that is lost, not to the amount of bicarbonate that is gained in the extracellular fluid. In other words, because there is a high blood pH, the bicarbonate distribution will be smaller than predicted by the 0.5 noted in the equation above. Since the buffer capacity of bicarbonate is greater than predicted for pH of 7.4, the bicarbonate rise in blood will be greater than the actual loss of hydrogen ion.
Don’t Miss: Ginger Tea Dissolves Kidney Stones
Ammoniagenesis And Nh4+ Excretion
An important aspect of renal acid-base physiology is the production and excretion of NH4+. shows this process. The kidney takes glutamine and metabolizes it to two molecules each of NH4+ and HCO3. The NH4+ is excreted into the urine, and the HCO3, which is new HCO3, is returned to the blood, where it replaces the HCO3 lost earlier in the titration of nonvolatile acids. also shows the fate of the NH4+ that is returned to the blood rather than being excreted in the urine. When this occurs, the NH4+ is converted to urea by the liver, and, in that process, H+ is generated. This H+ is buffered by HCO3 and thus negates the process of renal new HCO3 generation. Thus, from the perspective of renal acid-base physiology, NH4+ produced by the kidney must be excreted into the urine and not returned to the blood. For every milliequivalent of NH4+ excreted, a milliequivalent of new HCO3 is returned to the blood. This process accounts for approximately two-thirds of RNAE.
Fig. 6.General scheme for the production of HCO3 and NH4+ from the renal metabolism of glutamine. Also shown is the conversion of NH4+ to urea by the liver, which generates and H+ and thus consumes HCO3. See text for details.
Fig. 7.Renal handling of NH4+. Two mechanisms for the secretion of NH4+ by the collecting duct are shown. A: nonionic diffusion and diffusion trapping of NH3. B: secretion of NH4+ via Rh glycoprotein . See text for details.
Regulation Of Hco3 Reabsorption In The Proximal Tubule
A number of processes regulate proximal tubule HCO3 reabsorption both acutely and chronically. Many of these regulatory processes function to maintain acid-base homeostasis and are seemingly quite redundant this is true in both the proximal and distal nephron segments. However, other processes overlap with volume or sodium regulatory processes, and the acid-base effects seem secondary and at times, dysfunctional for pH per se for instance, during metabolic alkalosis induced by vomiting and volume depletion, various hormones are activated that restore volume status but secondarily maintain high plasma HCO3 and alkalemia.
Despite the regulation mentioned above, changes in proximal HCO3 reabsorption may not be directly reflected in changes in wholekidney net acid excretion or urinary HCO3 excretion, because additional HCO3 reabsorption and acid secretion occur in the distal nephron. Also, because virtually all of filtered HCO3 is normally reabsorbed by the kidneys, increases in HCO3 reabsorption per se cannot compensate for increased systemic acid loads This compensation can only occur with increased acid excretion as titratable acids or NH4+.
Read Also: What Std Messes With Your Kidneys
Generation Of Metabolic Alkalosis
Metabolic alkalosis may be generated by one of the following mechanisms:
- Loss of hydrogen ions
- Shift of hydrogen ions into the intracellular space
- Alkali administration
- Contraction alkalosis
Hydrogen ions may be lost through the kidneys or the GI tract. Vomiting or nasogastric suction generates metabolic alkalosis by the loss of gastric secretions, which are rich in hydrochloric acid . Whenever a hydrogen ion is excreted, a bicarbonate ion is gained in the extracellular space.
Renal losses of hydrogen ions occur whenever the distal delivery of sodium increases in the presence of excess aldosterone, which stimulates the electrogenic epithelial sodium channel in the collecting duct. As this channel reabsorbs sodium ions, the tubular lumen becomes more negative, leading to the secretion of hydrogen ions and potassium ions into the lumen.
Shift of hydrogen ions into the intracellular space mainly develops with hypokalemia. As the extracellular potassium concentration decreases, potassium ions move out of the cells. To maintain neutrality, hydrogen ions move into the intracellular space.
Administration of sodium bicarbonate in amounts that exceed the capacity of the kidneys to excrete this excess bicarbonate may cause metabolic alkalosis. This capacity is reduced when a reduction in filtered bicarbonate occurs, as observed in renal failure, or when enhanced tubular reabsorption of bicarbonate occurs, as observed in volume depletion .